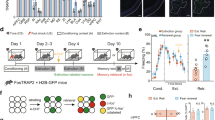
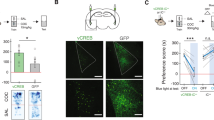
Abstract
The valence of memories is malleable because of their intrinsic reconstructive property1. This property of memory has been used clinically to treat maladaptive behaviours2. However, the neuronal mechanisms and brain circuits that enable the switching of the valence of memories remain largely unknown. Here we investigated these mechanisms by applying the recently developed memory engram cell- manipulation technique3,4. We labelled with channelrhodopsin-2 (ChR2) a population of cells in either the dorsal dentate gyrus (DG) of the hippocampus or the basolateral complex of the amygdala (BLA) that were specifically activated during contextual fear or reward conditioning. Both groups of fear-conditioned mice displayed aversive light-dependent responses in an optogenetic place avoidance test, whereas both DG- and BLA-labelled mice that underwent reward conditioning exhibited an appetitive response in an optogenetic place preference test. Next, in an attempt to reverse the valence of memory within a subject, mice whose DG or BLA engram had initially been labelled by contextual fear or reward conditioning were subjected to a second conditioning of the opposite valence while their original DG or BLA engram was reactivated by blue light. Subsequent optogenetic place avoidance and preference tests revealed that although the DG-engram group displayed a response indicating a switch of the memory valence, the BLA-engram group did not. This switch was also evident at the cellular level by a change in functional connectivity between DG engram-bearing cells and BLA engram-bearing cells. Thus, we found that in the DG, the neurons carrying the memory engram of a given neutral context have plasticity such that the valence of a conditioned response evoked by their reactivation can be reversed by re-associating this contextual memory engram with a new unconditioned stimulus of an opposite valence. Our present work provides new insight into the functional neural circuits underlying the malleability of emotional memory.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pavlov, I. P. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex (Oxford Univ. Press, 1927)
Wolpe, J. Psychotherapy by Reciprocal Inhibition (Stanford Univ. Press, 1958)
Liu, X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385 (2012)
Ramirez, S. et al. Creating a false memory in the hippocampus. Science 341, 387–391 (2013)
Muramoto, K., Ono, T., Nishijo, H. & Fukuda, M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience 52, 621–636 (1993)
Schoenbaum, G., Chiba, A. A. & Gallagher, M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J. Neurosci. 19, 1876–1884 (1999)
Paton, J. J., Belova, M. A., Morrison, S. E. & Salzman, C. D. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature 439, 865–870 (2006)
Shabel, S. J. & Janak, P. H. Substantial similarity in amygdala neuronal activity during conditioned appetitive and aversive emotional arousal. Proc. Natl Acad. Sci. USA 106, 15031–15036 (2009)
Amano, T., Duvarci, S., Popa, D. & Paré, D. The fear circuit revisited: contributions of the basal amygdala nuclei to conditioned fear. J. Neurosci. 31, 15481–15489 (2011)
Sangha, S., Chadick, J. Z. & Janak, P. H. Safety encoding in the basal amygdala. J. Neurosci. 33, 3744–3751 (2013)
Teyler, T. J. & DiScenna, P. The hippocampal memory indexing theory. Behav. Neurosci. 100, 147–154 (1986)
Rudy, J. W. & O’Reilly, R. C. Contextual fear conditioning, conjunctive representations, pattern completion, and the hippocampus. Behav. Neurosci. 113, 867–880 (1999)
Reijmers, L. G., Perkins, B. L., Matsuo, N. & Mayford, M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233 (2007)
Schiltz, C. A., Bremer, Q. Z., Landry, C. F. & Kelley, A. E. Food-associated cues alter forebrain functional connectivity as assessed with immediate early gene and proenkephalin expression. BMC Biol. 5, 16 (2007)
Wheeler, A. L. et al. Identification of a functional connectome for long-term fear memory in mice. PLOS Comput. Biol. 9, e1002853 (2013)
Kim, J., Kwon, J. T., Kim, H. S., Josselyn, S. A. & Han, J. H. Memory recall and modifications by activating neurons with elevated CREB. Nature Neurosci. 17, 65–72 (2014)
Huff, M. L., Miller, R. L., Deisseroth, K., Moorman, D. E. & LaLumiere, R. T. Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc. Natl Acad. Sci. USA 110, 3597–3602 (2013)
Malkesman, O. et al. The female urine sniffing test: a novel approach for assessing reward-seeking behavior in rodents. Biol. Psychiatry 67, 864–871 (2010)
Kiyokawa, Y., Hiroshima, S., Takeuchi, Y. & Mori, Y. Social buffering reduces male rats’ behavioral and corticosterone responses to a conditioned stimulus. Horm. Behav. 65, 114–118 (2014)
Maren, S. & Fanselow, M. S. Synaptic plasticity in the basolateral amygdala induced by hippocampal formation stimulation in vivo. J. Neurosci. 15, 7548–7564 (1995)
Maren, S. & Hobin, J. A. Hippocampal regulation of context-dependent neuronal activity in the lateral amygdala. Learn. Mem. 14, 318–324 (2007)
Herry, C. et al. Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606 (2008)
Stuber, G. D. et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475, 377–380 (2011)
Han, J. H. et al. Neuronal competition and selection during memory formation. Science 316, 457–460 (2007)
Choi, G. B. et al. Driving opposing behaviors with ensembles of piriform neurons. Cell 146, 1004–1015 (2011)
Franklin, K. B. J. & Paxinos, G. The Mouse Brain in Stereotaxic Coordinates (Academic Press, 2008)
Calcagnetti, D. J. & Schechter, M. D. Nicotine place preference using the biased method of conditioning. Prog. Neuropsychopharmacol. Biol. Psychiatry 18, 925–933 (1994)
Blumstein, D. T. & Daniel, J. C. Quantifying Behavior the JWatcher Way (Sinauer Associates, 2007)
Acknowledgements
We thank X. Zhou, C. Potter, D. Plana, J. Martin, M. Tsitsiklis, H. Sullivan, W. Yu and A. Moffa for help with the experiments; K. L. Mulroy, T. Ryan and D. Roy for comments and discussions on the manuscript, and all the members of the Tonegawa laboratory for their support. This work was supported by the funds from the RIKEN Brain Science Institute, the Howard Hughes Medical Institute and The JPB Foundation to S.T., and the National Institutes of Health Pre-doctoral Training Grant T32GM007287 to J.K.
Author information
Authors and Affiliations
Contributions
R.L.R., J.K. and S.T. contributed to the study design. R.L.R., J.K. and S.R. contributed to the data collection. X.L. cloned all constructs. R.L.R., J.K. and A.L.A. conducted the surgeries. R.L.R. and J.K. conducted the behavioural experiments. R.L.R. conducted the functional connectivity experiments. J.K. conducted the reversal experiments. R.L.R. contributed to the setup of the behavioural and optogenetic apparatus and programmed the behavioural software to run the experiments. R.L.R., J.K. and S.T. wrote the paper. All authors discussed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
Extended Data Figure 1 Light-induced avoidance and preference tests.
a, b, Shock place avoidance test. a, After the 0–3 min baseline (BSL), the most preferred zone was established as the target zone. Wild-type, shock mice (n = 11) received foot shocks (0.15 mA DC (direct current), 2 s duration every 5 s) when entering the target zone during the on phases (3–6 and 9–12 min). No shocks were delivered during the off phase (6–9 min). Wild-type, no shock mice (n = 24) received no shocks. b, The difference score was lower in the wild-type, shock mice compared to wild-type, no shock (t41 = 3.76, P < 0.001). c, d, Optogenetic place avoidance (OptoPA) test. c, Mice were allowed to explore the arena during baseline and the preferred side determined as the target zone. Light stimulation (20 Hz, 15 ms pulse width, 473 nm, > 10 mW) was applied when the mice entered the target zone during the on phases. Light stimulation was not delivered during the off phase. d, The difference score was lower in the DG ChR2 mice (n = 48) compared to DG mCherry-only mice (n = 39) (t85 = 3.55, P < 0.001). e, f, Female place preference test. e, After the 0–3 min baseline, the less preferred zone was established as the target zone. During the on phases (3–6 and 9–12 min), a corral containing a female was placed in the less preferred zone (target zone) and an empty corral was placed on the opposite side. Corrals, and the female, were removed during the off phase. f, Mice (n = 24) increased the time spent in the target zone compared to mice presented with two empty receptacles (n = 8) (t30 = 2.81, P < 0.01). g, h, Optogenetic place preference (OptoPP) test. g, Reward-labelled mice were allowed to explore the arena during baseline and the less preferred zone designated as the target zone. Light stimulation (20 Hz, 15 ms pulse width, 473 nm, > 10 mW) was applied while the subject was in the target zone of the chamber at the 3–6 min and 9–12 min epochs (on phase). h, The difference score was increased in the DG ChR2 mice (n = 54) compared to DG mCherry-only mice (n = 36) (t88 = 4.361, P < 0.001). a, c, e, g (right panel), Representative tracks for experimental animals. Dots mark the position of the animal every 5 video frames and accumulate where the mice spend more time.
Extended Data Figure 2 Light stimulation in the OptoPA and OptoPP tests has no effect during habituation.
a, Day 1 OptoPA habituation. There are no within group differences in the average duration spent in the target zone during the average of the baseline and off phases (off) and the averages of the two light on phases (on) during day 1 habituation in the OptoPA test, even though there is an overall difference between the two types of phases (F1,173 = 6.46, P < 0.05, 6 NS multiple comparisons. DG ChR2 n = 48; DG mCherry-only n = 39; DG ChR2, no US on day 3 n = 17; BLA ChR2 n = 32; BLA mCherry-only n = 27; BLA ChR2, no US on day 3 n = 27). b, Day 1 OptoPP habituation. There are no within group differences in the average time duration spent in the target zone during the average of the baseline and off phases (off) and the average of the two light on phases (on) during day 1 habituation in the OptoPP test, even though there is an overall difference between the two types of phases (F1,195 = 8.06, P < 0.01, 6 NS multiple comparisons. DG ChR2 n = 54; DG mCherry-only n = 36; DG ChR2, no US on day 3 n = 24; BLA ChR2 n = 35; BLA mCherry-only n = 31; BLA ChR2, no US on day 3 n = 21). c, d, There are no differences between experimental groups and wild-type mice tested without light stimulation. c, In the OptoPA test, the difference scores (on minus off) are similar between experimental groups (same as panel a) and wild-type mice (n = 33) that did not receive light stimulation (F6,205 = 0.19, NS). d, In the OptoPP test, the difference scores (on minus off) are similar between experimental groups (same as panel b) and wild-type mice (n = 33) that did not receive light stimulation (F6,227 = 0.21, NS).
Extended Data Figure 3 Fibre positions in DG and BLA.
a, Representative example of the fibre location and the expression of the ChR2–mCherry construct in the DG. b, Representative example of the fibre location and the expression of the ChR2–mCherry construct in the BLA.
Rights and permissions
About this article
Cite this article
Redondo, R., Kim, J., Arons, A. et al. Bidirectional switch of the valence associated with a hippocampal contextual memory engram. Nature 513, 426–430 (2014). https://doi.org/10.1038/nature13725
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13725
This article is cited by
-
Engram neurons: Encoding, consolidation, retrieval, and forgetting of memory
Molecular Psychiatry (2023)
-
Locus coeruleus input-modulated reactivation of dentate gyrus opioid-withdrawal engrams promotes extinction
Neuropsychopharmacology (2023)
-
Mapping the spatial transcriptomic signature of the hippocampus during memory consolidation
Nature Communications (2023)
-
Closed-loop brain stimulation augments fear extinction in male rats
Nature Communications (2023)
-
ACx-projecting cholinergic neurons in the NB influence the BLA ensembles to modulate the discrimination of auditory fear memory
Translational Psychiatry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.