Abstract
How does the mammalian retina detect motion? This classic problem in visual neuroscience has remained unsolved for 50 years. In search of clues, here we reconstruct Off-type starburst amacrine cells (SACs) and bipolar cells (BCs) in serial electron microscopic images with help from EyeWire, an online community of ‘citizen neuroscientists’. On the basis of quantitative analyses of contact area and branch depth in the retina, we find evidence that one BC type prefers to wire with a SAC dendrite near the SAC soma, whereas another BC type prefers to wire far from the soma. The near type is known to lag the far type in time of visual response. A mathematical model shows how such ‘space–time wiring specificity’ could endow SAC dendrites with receptive fields that are oriented in space–time and therefore respond selectively to stimuli that move in the outward direction from the soma.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Borst, A. & Euler, T. Seeing things in motion: models, circuits, and mechanisms. Neuron 71, 974–994 (2011)
Vaney, D. I., Sivyer, B. & Taylor, W. R. Direction selectivity in the retina: symmetry and asymmetry in structure and function. Nature Rev. Neurosci. 13, 194–208 (2012)
Euler, T., Detwiler, P. B. & Denk, W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418, 845–852 (2002)
Hausselt, S. E., Euler, T., Detwiler, P. B. & Denk, W. A dendrite-autonomous mechanism for direction selectivity in retinal starburst amacrine cells. PLoS Biol. 5, e185 (2007)
Yonehara, K. et al. The first stage of cardinal direction selectivity is localized to the dendrites of retinal ganglion cells. Neuron 79, 1078–1085 (2013)
Wässle, H., Puller, C., Müller, F. & Haverkamp, S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J. Neurosci. 29, 106–117 (2009)
Baden, T., Berens, P., Bethge, M. & Euler, T. Spikes in mammalian bipolar cells support temporal layering of the inner retina. Curr. Biol. 23, 48–52 (2013)
Borghuis, B. G., Marvin, J. S., Looger, L. L. & Demb, J. B. Two-photon imaging of nonlinear glutamate release dynamics at bipolar cell synapses in the mouse retina. J. Neurosci. 33, 10972–10985 (2013)
Briggman, K. L., Helmstaedter, M. & Denk, W. Wiring specificity in the direction-selectivity circuit of the retina. Nature 471, 183–188 (2011)
Lintott, C. J. et al. Galaxy Zoo: morphologies derived from visual inspection of galaxies from the Sloan Digital Sky Survey. Mon. Not. R. Astron. Soc. 389, 1179–1189 (2008)
Cooper, S. et al. Predicting protein structures with a multiplayer online game. Nature 466, 756–760 (2010)
Fiala, J. C. Reconstruct: a free editor for serial section microscopy. J. Microsc. 218, 52–61 (2005)
Von Ahn, L. & Dabbish, L. Labeling images with a computer game. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems 319–326 (ACM, 2004)
Helmstaedter, M. et al. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature 500, 168–174 (2013)
Famiglietti, E. V. Synaptic organization of starburst amacrine cells in rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. J. Comp. Neurol. 309, 40–70 (1991)
Berry, M. J., II & Meister, M. Refractoriness and neural precision. J. Neurosci. 18, 2200–2211 (1998)
Baccus, S. A. & Meister, M. Fast and slow contrast adaptation in retinal circuitry. Neuron 36, 909–919 (2002)
Watson, A. B. & Ahumada, A. J., Jr Model of human visual-motion sensing. J. Opt. Soc. Am. A 2, 322–341 (1985)
Adelson, E. H. & Bergen, J. R. Spatiotemporal energy models for the perception of motion. J. Opt. Soc. Am. A 2, 284–299 (1985)
Reichardt, W. in Sensory Communication (ed. Rosenblith, W. A. ) 303–317 (MIT Press, 1961)
Barlow, H. B. & Levick, W. R. The mechanism of directionally selective units in rabbit’s retina. J. Physiol. (Lond.) 178, 477–504 (1965)
Borst, A., Reisenman, C. & Haag, J. Adaptation of response transients in fly motion vision. II: model studies. Vision Res. 43, 1311–1324 (2003)
Lagnado, L., Gomis, A. & Job, C. Continuous vesicle cycling in the synaptic terminal of retinal bipolar cells. Neuron 17, 957–967 (1996)
Tukker, J. J., Taylor, W. R. & Smith, R. G. Direction selectivity in a model of the starburst amacrine cell. Vis. Neurosci. 21, 611–625 (2004)
Borg-Graham, L. J. & Grzywacz, N. M. in Single Neuron Computation (eds McKenna, T., Davis, J. & Zornetzer, S. F. ) Ch. 13 347–76 (Academic San Diego, 1992)
Gavrikov, K. E., Dmitriev, A. V., Keyser, K. T. & Mangel, S. C. Cation–chloride cotransporters mediate neural computation in the retina. Proc. Natl Acad. Sci. USA 100, 16047–16052 (2003)
Münch, T. A. & Werblin, F. S. Symmetric interactions within a homogeneous starburst cell network can lead to robust asymmetries in dendrites of starburst amacrine cells. J. Neurophysiol. 96, 471–477 (2006)
Lee, S. & Zhou, Z. J. The synaptic mechanism of direction selectivity in distal processes of starburst amacrine cells. Neuron 51, 787–799 (2006)
Kim, I.-J., Zhang, Y., Yamagata, M., Meister, M. & Sanes, J. R. Molecular identification of a retinal cell type that responds to upward motion. Nature 452, 478–482 (2008)
Maisak, M. S. et al. A directional tuning map of Drosophila elementary motion detectors. Nature 500, 212–216 (2013)
Takemura, S. Y. et al. A visual motion detection circuit suggested by Drosophila connectomics. Nature 500, 175–181 (2013)
Braitenberg, V. & Schüz, A. Cortex: Statistics and Geometry of Neuronal Connectivity 2nd edn (Springer Berlin, 1998)
Kalisman, N., Silberberg, G. & Markram, H. Deriving physical connectivity from neuronal morphology. Biol. Cybern. 88, 210–218 (2003)
Binzegger, T., Douglas, R. J. & Martin, K. A. C. A quantitative map of the circuit of cat primary visual cortex. J. Neurosci. 24, 8441–8453 (2004)
Stepanyants, A. & Chklovskii, D. B. Neurogeometry and potential synaptic connectivity. Trends Neurosci. 28, 387–394 (2005)
Fried, S. I., Münch, T. A. & Werblin, F. S. Mechanisms and circuitry underlying directional selectivity in the retina. Nature 420, 411–414 (2002)
Yonehara, K. et al. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature 469, 407–410 (2010)
Wei, W., Hamby, A. M., Zhou, K. & Feller, M. B. Development of asymmetric inhibition underlying direction selectivity in the retina. Nature 469, 402–406 (2011)
Surowiecki, J. The Wisdom of Crowds (Anchor, 2005)
Turaga, S., Briggman, K., Helmstaedter, M., Denk, W. & Seung, H. S. in Advances in Neural Information Processing Systems 22. 1865–1873 (2009)
Turaga, S. C. et al. Convolutional networks can learn to generate affinity graphs for image segmentation. Neural Comput. 22, 511–538 (2010)
Ciresan, D. et al. in Advances in Neural Information Processing Systems 25. 2852–2860 (2012)
Park, S. J. H., Kim, I. -J., Looger, L. L., Demb, J. B. & Borghuis, B. G. Excitatory synaptic inputs to mouse on-off direction-selective retinal ganglion cells lack direction tuning. J. Neurosci. 34, 3976–3981 (2014)
Mutch, J., Knoblich, U. & Poggio, T. CNS: a GPU-Based Framework for Simulating Cortically-Organized Networks Tech. Rep. MIT-CSAIL-TR-2010–013/CBCL-286 (MIT, 2010)
Jeon, C.-J., Strettoi, E. & Masland, R. H. The major cell populations of the mouse retina. J. Neurosci. 18, 8936–8946 (1998)
Snyder, J. P. Map Projections–A working Manual 1395 (USGPO, 1987)
Acknowledgements
This research was made possible by funding from the Gatsby Charitable Foundation, the Howard Hughes Medical Institute, the Human Frontier Science Program, an anonymous donor, and the National Institutes of Health. K.L. was supported by a Samsung Scholarship. Support from the AWS Research Grants Program gave EyeWire global reach through Amazon Cloudfront. We thank K. Briggman for providing the e2198 data set. J. Mutch created the CNS framework on which CNPKG is based. D. Jia, R. Shearer, and B. Warne assisted in early stages of software development, and W. Silversmith with recent modifications. R. Prentki, L. Trawinski, M. Sorek, A. Ostojic, C. David, R. Avery, S. Temple, A. Bost, M. Greenstein and M. Evans worked in the laboratory to reconstruct neurons, and the first six also served as GrimReaper and hosted EyeWire competitions. Additional reconstructions were provided by R. Han, M. Gavrin, G. Lu, A. Ortiz and D. Udvary. All were trained by R. Prentki, who also created training videos for EyeWirers. We are grateful to A. Norton for 3D renderings, and to E. Almeida for EyeWire graphics. We acknowledge discussions with T. Baden, M. Berry, B. Borghuis, A. Borst, E. J. Chichilnisky, D. Chklovskii, D. Clark, J. Demb, T. Euler, M. Helmstaedter, A. Huberman, S. Lee, R. Masland, J. Sanes and Z. Zhou.
Author information
Authors and Affiliations
Consortia
Contributions
J.S.K. created algorithms, software and procedures for crowd intelligence and learning, and applied them to generate neuron reconstructions. J.S.K. and M.J.G. classified bipolar cells. M.J.G. analysed contact and co-stratification, aided by code from A.Z. and input from W.D. H.S.S. devised the model with help from B.F.B. and M.C. S.C.T. trained the convolutional network. M.P. and M.B. implemented software and algorithms created by A.Z. for interactive segmentation and 3D visualization, with guidance from S.C.T. M.R. created the EyeWire game and M.B. its data infrastructure. K.L. quantified EyeWirer accuracy and learning. A.R. mobilized and studied the EyeWire community. EyeWirers reconstructed neurons and built extensions to EyeWire. H.S.S. wrote the paper with help from J.S.K., M.J.G. and A.R.
Corresponding author
Ethics declarations
Competing interests
W.D. receives license income for SBEM technology from Gatan Inc.
Additional information
Extended data figures and tables
Extended Data Figure 1 EyeWire screenshots.
a, Numerical score after gameplay of a cube, with leaderboard below. b, Overview mode with neuron under reconstruction (centre), global chat (bottom left), progress bar for neuron (top left), leaderboard (right), settings and help (bottom right). c, Tutorial play.
Extended Data Figure 3 EyeWire demographics.
a, b, Data based on 729 responses to the questionnaire in Extended Data Fig. 2. Age distribution of (a) all respondents and (b) those among the top 100 players ranked by number of cubes submitted. c, Gender distribution of all respondents and those among the top 100 players. d, Distribution of educational levels.
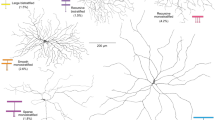
Extended Data Figure 4 Entirety of reconstructed SACs.
Only the central region of this plexus of SAC dendrites is portrayed in Fig. 3b. Scale bar, 50 μm.
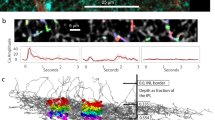
Extended Data Figure 5 Clustering procedure for BCs.
a, Cells were divided by the 75th percentile of their stratification profiles. b, The shallow cluster BC1/2 was separated into BC1 and BC2 using stratification width, defined as the difference between 75th and 25th percentiles. c, The deep cluster BC3/4 was divided by 10th percentile into BC4 and BC3. d, BC3 was divided by axonal volume to yield BC3a and BC3b. Scatter plots of the BC1/2 (e) and BC3/4 (f) divisions show swaps made to eliminate mosaic violations. No swaps between BC1/2 and BC3/4 were needed.
Extended Data Figure 6 Mosaics of Off BC types.
a–e, Reconstructed BCs of types 1, 2, 3a, 3b and 4 (a through e, respectively). BC1/2 mosaics appear complete. BC3/4 mosaics show some gaps, probably because some thin axons were missed in the INL (Methods). Scale bar, 50 μm. f, Statistics of BC types. Means and standard deviation of the hull area (area of the convex hull around the cell) are in μm2. Type densities are the number of cells (n) divided by the area of the union of hulls of that cell type, and are in cells per mm2 without compensation for tissue shrinkage (Methods). Our densities resemble those of Wässle et al.6, who found 2,233, 3,212, 1,866, 3,254 and 3,005 cells per mm2.
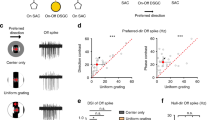
Extended Data Figure 7 Alternative contact analysis.
Analysis based on summing over BC–SAC pairs rather than averaging as in the main text. a, Total BC–SAC contact versus distance from the SAC soma. b, Total SAC area within the union of convex hulls of each BC type versus distance. The peak at 80 μm is the location of maximum dendritic branching. The sharp decrease at larger distances is due to thinning and termination of branches. The graphs differ across BC types, which in our sample do not cover exactly the same retinal areas. c, Fraction of SAC area in contact with BC types, estimated by dividing contact area (a) by SAC area (b). This estimate is similar to that of Fig. 4d, but lacks error bars. d, Fraction of SAC area contacted by all BC types, the sum of the contact fractions in c. Also plotted is the contact predicted by co-stratification, the sum of the curves from Fig. 5b.
Extended Data Figure 8 Proximity versus contact.
Neurons that intermingle may or may not contact each other. a, b, Type 2 (a) and 3a BCs (b) contacting SACs. The cells are roughly 24 and 21 μm wide, respectively. c, d, Other SACs are well within the arborizations of the same two BCs, yet make no contact at all.
Extended Data Figure 9 Model direction selectivity index (DSI) versus stimulus speed.
The graphs are for travelling sine waves of various wavelengths λ (units of Δx). Speed is in units of Δx/τ. The preferred speed (horizontal location of each peak) is λ/(2π). Note that responses are cut off at high speeds by the temporal filters of the model, but the DSI can decay more slowly.
Supplementary information
Supplementary Information
This file contains Supplementary Equations showing detailed derivation of the mathematical model of direction selectivity, calculation of the direction selectivity index for a special case that does not depend on the detailed forms of the filters, comparison with experiments, and cable theory estimate of dendritic conduction time. It also contains Supplementary Notes, which include EyeWire demographics, community structure, competitions, and list of EyeWirers who reconstructed SACs. (PDF 161 kb)
Off SAC with BC2 and BC3a axons.
Off SAC with BC2 and BC3a axons. Off SAC in red, BC2 axon in yellow, and BC3a axon in blue. (MOV 19301 kb)
Rights and permissions
About this article
Cite this article
Kim, J., Greene, M., Zlateski, A. et al. Space–time wiring specificity supports direction selectivity in the retina. Nature 509, 331–336 (2014). https://doi.org/10.1038/nature13240
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13240
This article is cited by
-
A presynaptic source drives differing levels of surround suppression in two mouse retinal ganglion cell types
Nature Communications (2024)
-
Distributed feature representations of natural stimuli across parallel retinal pathways
Nature Communications (2024)
-
RoboEM: automated 3D flight tracing for synaptic-resolution connectomics
Nature Methods (2024)
-
Dendritic mGluR2 and perisomatic Kv3 signaling regulate dendritic computation of mouse starburst amacrine cells
Nature Communications (2024)
-
Online citizen science with the Zooniverse for analysis of biological volumetric data
Histochemistry and Cell Biology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.