Abstract
Efferocytosis, the process by which dying or dead cells are removed by phagocytosis, has an important role in development, tissue homeostasis and innate immunity1. Efferocytosis is mediated, in part, by receptors that bind to exofacial phosphatidylserine (PS) on cells or cellular debris after loss of plasma membrane asymmetry. Here we show that a bacterial pathogen, Listeria monocytogenes, can exploit efferocytosis to promote cell-to-cell spread during infection. These bacteria can escape the phagosome in host cells by using the pore-forming toxin listeriolysin O (LLO) and two phospholipase C enzymes2. Expression of the cell surface protein ActA allows L. monocytogenes to activate host actin regulatory factors and undergo actin-based motility in the cytosol, eventually leading to formation of actin-rich protrusions at the cell surface. Here we show that protrusion formation is associated with plasma membrane damage due to LLO’s pore-forming activity. LLO also promotes the release of bacteria-containing protrusions from the host cell, generating membrane-derived vesicles with exofacial PS. The PS-binding receptor TIM-4 (encoded by the Timd4 gene) contributes to efficient cell-to-cell spread by L. monocytogenes in macrophages in vitro and growth of these bacteria is impaired in Timd4−/− mice. Thus, L. monocytogenes promotes its dissemination in a host by exploiting efferocytosis. Our results indicate that PS-targeted therapeutics may be useful in the fight against infections by L. monocytogenes and other bacteria that use similar strategies of cell-to-cell spread during infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Ravichandran, K. S. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity 35, 445–455 (2011)
Mostowy, S. & Cossart, P. Virulence factors that modulate the cell biology of Listeria infection and the host response. Adv. Immunol. 113, 19–32 (2012)
Robbins, J. R. et al. Listeria monocytogenes exploits normal host cell processes to spread from cell to cell. J. Cell Biol. 146, 1333–1350 (1999)
Alberti-Segui, C., Goeden, K. R. & Higgins, D. E. Differential function of Listeria monocytogenes listeriolysin O and phospholipases C in vacuolar dissolution following cell-to-cell spread. Cell. Microbiol. 9, 179–195 (2007)
Gedde, M. M., Higgins, D. E., Tilney, L. G. & Portnoy, D. A. Role of listeriolysin O in cell-to-cell spread of Listeria monocytogenes . Infect. Immun. 68, 999–1003 (2000)
Schnupf, P. & Portnoy, D. A. Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 9, 1176–1187 (2007)
Glomski, I. J., Gedde, M. M., Tsang, A. W., Swanson, J. A. & Portnoy, D. A. The Listeria monocytogenes hemolysin has an acidic pH optimum to compartmentalize activity and prevent damage to infected host cells. J. Cell Biol. 156, 1029–1038 (2002)
Nomura, T. et al. Irreversible loss of membrane-binding activity of Listeria-derived cytolysins in non-acidic conditions: a distinct difference from allied cytolysins produced by other Gram-positive bacteria. Microbiology 153, 2250–2258 (2007)
Schnupf, P., Portnoy, D. A. & Decatur, A. L. Phosphorylation, ubiquitination and degradation of listeriolysin O in mammalian cells: role of the PEST-like sequence. Cell. Microbiol. 8, 353–364 (2006)
Hamon, M. A., Ribet, D., Stavru, F. & Cossart, P. Listeriolysin O: the Swiss army knife of Listeria . Trends Microbiol. 20, 360–368 (2012)
Cassidy, S. K. et al. Membrane damage during Listeria monocytogenes infection triggers a caspase-7 dependent cytoprotective response. PLoS Pathog. 8, e1002628 (2012)
Idone, V., Tam, C. & Andrews, N. W. Two-way traffic on the road to plasma membrane repair. Trends Cell Biol. 18, 552–559 (2008)
Gründling, A., Gonzalez, M. D. & Higgins, D. E. Requirement of the Listeria monocytogenes broad-range phospholipase PC-PLC during infection of human epithelial cells. J. Bacteriol. 185, 6295–6307 (2003)
Draeger, A., Monastyrskaya, K. & Babiychuk, E. B. Plasma membrane repair and cellular damage control: the annexin survival kit. Biochem. Pharmacol. 81, 703–712 (2011)
Fadeel, B. & Xue, D. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Crit. Rev. Biochem. Mol. Biol. 44, 264–277 (2009)
Waite, J. C. et al. Dynamic imaging of the effector immune response to Listeria infection in vivo . PLoS Pathog. 7, e1001326 (2011)
Pust, S., Morrison, H., Wehland, J., Sechi, A. S. & Herrlich, P. Listeria monocytogenes exploits ERM protein functions to efficiently spread from cell to cell. EMBO J. 24, 1287–1300 (2005)
Babiychuk, E. B., Monastyrskaya, K., Potez, S. & Draeger, A. Blebbing confers resistance against cell lysis. Cell Death Differ. 18, 80–89 (2011)
Keyel, P. A. et al. Streptolysin O clearance through sequestration into blebs that bud passively from the plasma membrane. J. Cell Sci. 124, 2414–2423 (2011)
Feng, D. et al. Cellular internalization of exosomes occurs through phagocytosis. Traffic 11, 675–687 (2010)
Miyanishi, M. et al. Identification of Tim4 as a phosphatidylserine receptor. Nature 450, 435–439 (2007)
Rodriguez-Manzanet, R. et al. T and B cell hyperactivity and autoimmunity associated with niche-specific defects in apoptotic body clearance in TIM-4-deficient mice. Proc. Natl Acad. Sci. USA 107, 8706–8711 (2010)
Martin, C. J. et al. Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe 12, 289–300 (2012)
Appelberg, R. & Leal, I. S. Mutants of Listeria monocytogenes defective in in vitro invasion and cell-to-cell spreading still invade and proliferate in hepatocytes of neutropenic mice. Infect. Immun. 68, 912–914 (2000)
Drevets, D. A. Dissemination of Listeria monocytogenes by infected phagocytes. Infect. Immun. 67, 3512–3517 (1999)
Friedrich, N., Hagedorn, M., Soldati-Favre, D. & Soldati, T. Prison break: pathogens’ strategies to egress from host cells. Microbiol. Mol. Biol. Rev. 76, 707–720 (2012)
Meertens, L. et al. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe 12, 544–557 (2012)
Mercer, J. & Helenius, A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science 320, 531–535 (2008)
Hagedorn, M., Rohde, K. H., Russell, D. G. & Soldati, T. Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science 323, 1729–1733 (2009)
Hybiske, K. & Stephens, R. S. Mechanisms of host cell exit by the intracellular bacterium Chlamydia . Proc. Natl Acad. Sci. USA 104, 11430–11435 (2007)
Bishop, D. K. & Hinrichs, D. J. Adoptive transfer of immunity to Listeria monocytogenes. The influence of in vitro stimulation on lymphocyte subset requirements. J. Immunol. 139, 2005–2009 (1987)
Jones, S. & Portnoy, D. A. Characterization of Listeria monocytogenes pathogenesis in a strain expressing perfringolysin O in place of listeriolysin O. Infect. Immun. 62, 5608–5613 (1994)
Lauer, P., Chow, M. Y., Loessner, M. J., Portnoy, D. A. & Calendar, R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J. Bacteriol. 184, 4177–4186 (2002)
Skoble, J., Portnoy, D. A. & Welch, M. D. Three regions within ActA promote Arp2/3 complex-mediated actin nucleation and Listeria monocytogenes motility. J. Cell Biol. 150, 527–538 (2000)
Smith, G. A. et al. The two distinct phospholipases C of Listeria monocytogenes have overlapping roles in escape from a vacuole and cell-to-cell spread. Infect. Immun. 63, 4231–4237 (1995)
Shen, A. & Higgins, D. E. The 5′ untranslated region-mediated enhancement of intracellular listeriolysin O production is required for Listeria monocytogenes pathogenicity. Mol. Microbiol. 57, 1460–1473 (2005)
Rodriguez-Manzanet, R. et al. TIM-4 expressed on APCs induces T cell expansion and survival. J. Immunol. 180, 4706–4713 (2008)
Munsie, L. N., Caron, N., Desmond, C. R. & Truant, R. Lifeact cannot visualize some forms of stress-induced twisted F-actin. Nature Methods 6, 317 (2009)
Brumell, J. H., Rosenberger, C. M., Gotto, G. T., Marcus, S. L. & Finlay, B. B. SifA permits survival and replication of Salmonella typhimurium in murine macrophages. Cell. Microbiol. 3, 75–84 (2001)
Acknowledgements
We are grateful to S. Gray-Owen, S. Grinstein and D. Portnoy for providing reagents and advice and to D. Holmyard for help with electron microscopy. J.H.B. holds the Pitblado Chair in Cell Biology. Infrastructure for the Brumell laboratory was provided by a Leader’s Opportunity Fund grant from the Canadian Foundation for Innovation and the Ontario Innovation Trust. R.F. was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research in partnership with the Canadian Association of Gastroenterology and the Crohn’s and Colitis Foundation of Canada. S.O. was supported by a postdoctoral fellowship from the Research Training Committee at the Hospital for Sick Children. This work was supported by an operating grant from The Arthritis Society of Canada (#RG11/013) to J.H.B. and a US Public Health Service grant (AI053669) from the National Institutes of Health to D.E.H.
Author information
Authors and Affiliations
Contributions
J.H.B., M.A.C., S.O. and D.E.H. designed the experiments and wrote the paper. M.A.C., R.F., J.M.v.R., V.C. and S.O. performed the experiments. A.M.M. and V.K.K. contributed reagents and consultations.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Extended data figures and tables
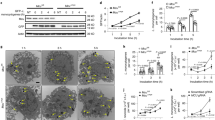
Extended Data Figure 1 Annexins promote membrane repair during L. monocytogenes infection.
a, HeLa cells were treated with the indicated siRNA for 48 h and then infected with wild-type L. monocytogenes at an MOI of 100. At 6 h post-infection, medium was switched to Tyrodes buffer containing 0.5 mg ml−1 PI with or without calcium. Cells were fixed at 60 min after PI addition and then stained for bacteria and DNA (DAPI). The percentage of 100 random infected cells that were PI+ cells were enumerated by microscopic analysis. Averages ± s.d. for three independent experiments are shown. P values were calculated using one-way ANOVA. *P < 0.05. b, Knockdown of gene expression by siRNA was confirmed by western blotting. Images are representative of two independent experiments. c, Recruitment of annexin A2 to PS+ structures containing bacteria. Boxes in low-magnification image indicate areas enlarged in bottom panels. Arrows indicate PS+ structures that co-localize with annexin A2. Images are representative of three independent experiments. Scale bars, 10 µm for low-magnification images, 2 µm for enlarged regions of interest.
Extended Data Figure 2 Actin-based motility promotes LLO-mediated membrane damage during L. monocytogenes infection.
a, HeLa cells were infected with the indicated L. monocytogenes strain. At 6 h post-infection, medium was switched to Tyrodes buffer containing 0.5 mg ml−1 PI with or without calcium. Cells were fixed at 60 min after PI addition and then stained for bacteria and DNA (DAPI). Confocal images representative of three independent experiments are shown (n = 100). PI+ cells were enumerated and results are shown in Fig. 1d. Where indicated, uninfected cells were treated with saponin to permeabilize membranes and allow PI entry, serving as a positive control. Scale bars, 10 µm. b, HeLa cells were infected with wild-type L. monocytogenes and subjected to membrane damage assay as in a in the presence of either DMSO or the actin cytoskeleton inhibitors latrunculin B or cytochalasin D. Averages ± s.d. for three independent experiments are shown (n = 100). P values were calculated using two-tailed Student’s t-test. *P < 0.05.
Extended Data Figure 3 Annexin A5–Alexa 488 as a probe to label PS.
Live HeLa cells were cooled on ice and stained with a fluorescent probe (annexin A5–Alexa 488) for 10 min to label exofacial PS. Cells were then fixed and stained with phalloidin Alexa 568 to visualize F-actin. In uninfected control experiments, low amounts of exofacial PS was detected in the membranes of cells, due to asymmetry of PS distribution in the plasma membrane. By contrast, treatment of cells with the pore-forming surfactant saponin led to robust staining of cells with annexin A5–Alexa 488. Images representative of three independent experiments. Scale bars, 10 µm.
Extended Data Figure 4 Formation of PS+ structures during L. monocytogenes infection.
Low-magnification images used to generate images shown in Fig. 2a. HeLa cells were infected with wild-type L. monocytogenes expressing RFP for 6 h and then cooled on ice and stained with a fluorescent probe (annexin A5–Alexa 488) for 10 min to label exofacial PS. Cells were then fixed and analysed by fluorescence microscopy to identify PS+ structures and bacteria. SEM of the same cell revealed that PS+ structures were associated with the dorsal surface of infected cells. Differential interference contrast (DIC) microscopy of cells was also performed to help identify cells for correlative imaging analysis. Images are representative of two independent experiments. Scale bars, 20 µm.
Extended Data Figure 5 PS+ bacteria are present with a host-derived membrane structure.
a, HeLa cells were infected with wild-type L. monocytogenes expressing RFP for 8 h and then labelled with a probe for exofacial PS (annexin A5–Alexa 488). Cells were then rapidly stained with anti-Listeria antibodies (5 min) to label extracellular bacteria. Cells were then fixed and analysed by fluorescence microscopy to identify PS+ structures and bacteria. Bacteria that co-localize with exofacial PS but are not labelled with anti-Listeria antibodies in the extracellular medium are indicated with arrows. Extracellular bacteria do not label with annexin A5–Alexa 488, indicating that this probe does not bind non-specifically to bacteria. Box in low-magnification image indicates area enlarged in bottom panels. Images are representative of three independent experiments. Scale bars, 10 µm for low magnification, 2 µm for high magnification. b, Cells were infected and stained as in a and analysed by fluorescence microscopy. Bacteria that co-localize with exofacial PS were scored for their accessibility to anti-Listeria antibodies present in the extracellular medium. Data show that the majority of PS+ bacteria are not accessible to anti-Listeria antibodies. Averages ± s.d. for two independent experiments are shown (n = 100).
Extended Data Figure 6 Formation of PS+ structures during L. monocytogenes infection of epithelial cells and macrophages.
a, Henle-407 human intestinal epithelial cells were infected with wild-type L. monocytogenes for 6 h and then incubated with a probe for exofacial PS (annexin A5–Alexa 488; green). Cells were then fixed and stained with phalloidin to visualize F-actin (red) and bacteria (blue). Cells were analysed by fluorescence microscopy to identify PS+ structures and bacteria. Images are representative of three independent experiments. b, Mouse BMDMs from C57BL/6 mice were infected and stained as in a. Scale bars, 10 µm. Images are representative of three independent experiments.
Extended Data Figure 7 Release of PS+ structures containing L. monocytogenes from infected cells.
a, HeLa cells were infected with wild-type L. monocytogenes for 6 h and then incubated with a probe for exofacial PS (annexin A5–Alexa 488; green). Cells were then fixed and stained with phalloidin to visualize F-actin (red) and bacteria (blue). Cells were analysed by fluorescence microscopy to identify PS+ structures and bacteria. Inset shows PS+ bacteria that are not cell associated. Images are representative of three independent experiments. b, HeLa cells were infected with ΔplcAΔplcB mutant bacteria for 6 h. The supernatant from the infected cultures was then removed and centrifuged onto poly-l-lysine-coated coverslips. Bacteria associated with coverslips were then stained with a probe for exofacial PS (annexin A5–Alexa 488; green). Cells were then fixed and stained for bacteria (blue). Coverslips were analysed by fluorescence microscopy to identify PS+ bacteria. Inset shows PS+ bacteria. Scale bars, 10 µm for low-magnification images, 2 µm for insets. Images are representative of three independent experiments.
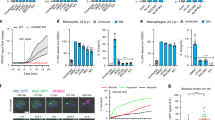
Extended Data Figure 8 Growth of L. monocytogenes in the cytosol of Timd4−/− macrophages is not impaired.
Gentamicin protection assay to measure intracellular bacterial growth. BMDMs were harvested from C57BL/6 or Timd4−/− mice and seeded at a density of 3 × 105 cells per well. Cells were then infected with wild-type L. monocytogenes in the presence of extracellular gentamicin. At the indicated times, cell lysates were plated and intracellular bacterial numbers (c.f.u.) were determined. Averages ± s.d. for two independent experiments are shown.
Extended Data Figure 9 Cytokine measurements.
a, Measurement of cytokines after in vitro infection of BMDMs from C57BL/6 or Timd4−/− mice with wild-type L. monocytogenes for the indicated time. Data from one of two independent experiments are shown. b, Measurement of basal cytokines in tissues of C57BL/6 or Timd4−/− mice without infection. Averages ± s.d. for three independent experiments are shown. P values were calculated using one-way ANOVA.
Extended Data Figure 10 L. monocytogenes exploits efferocytosis to promote cell-to-cell spread during infection.
Model shows the steps that promote cell-to-cell spread by L. monocytogenes. 1. Protrusion formation via actin-based motility. 2. LLO-mediated damage to the plasma membrane leads to loss of membrane asymmetry and exofacial PS on protrusions. The exofacial exposure of PS promotes protrusion association with neighbouring cells (right). 3. Loss of membrane asymmetry and PS exposure extends along length of protrusions. 4. Calcium entry activates membrane repair pathways that promote scission of the protrusion. Bacteria are released from the cell in PS+ vesicles. 5. Macrophages mediate uptake of PS+ vesicles containing bacteria via the PS-binding receptor TIM-4. PS+ vesicles may be engulfed by neighbouring cells either near the infected cell surface (left side) or within enclosed spaces that form as a result of protrusion penetration into the neighbouring cell (right side). TIM-4 may also promote L. monocytogenes infection indirectly, through its ability to suppress basal levels of pro-inflammatory cytokines as part of its homeostatic function in the immune system.
Supplementary information
Lm protrusion formation leads to exofacial PS exposure on membrane vesicles
Cells were transfected with LifeAct-RFP (red) and then infected with wild type Lm expressing GFP at an MOI of 100 for 6h. Live infected cells were then analyzed by spinning disk confocal microscopy with Annexin V-Alexa 647 in the medium to label exofacial PS (blue). Frames from this video were cropped and are presented in Figure 3B. Images are representative of 3 independent experiments. (MOV 113 kb)
Rights and permissions
About this article
Cite this article
Czuczman, M., Fattouh, R., van Rijn, J. et al. Listeria monocytogenes exploits efferocytosis to promote cell-to-cell spread. Nature 509, 230–234 (2014). https://doi.org/10.1038/nature13168
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature13168
This article is cited by
-
A patatin-like phospholipase mediates Rickettsia parkeri escape from host membranes
Nature Communications (2022)
-
Listeria exploits IFITM3 to suppress antibacterial activity in phagocytes
Nature Communications (2021)
-
Tim-4 functions as a scavenger receptor for phagocytosis of exogenous particles
Cell Death & Disease (2020)
-
The Tim gene family in efferocytosis
Genes & Genomics (2020)
-
Intracellular bacteria engage a STING–TBK1–MVB12b pathway to enable paracrine cGAS–STING signalling
Nature Microbiology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.