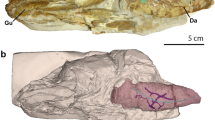
Abstract
The unidirectional airflow patterns in the lungs of birds have long been considered a unique and specialized trait associated with the oxygen demands of flying, their endothermic metabolism1 and unusual pulmonary architecture2,3. However, the discovery of similar flow patterns in the lungs of crocodilians indicates that this character is probably ancestral for all archosaurs—the group that includes extant birds and crocodilians as well as their extinct relatives, such as pterosaurs and dinosaurs4,5,6. Unidirectional flow in birds results from aerodynamic valves, rather than from sphincters or other physical mechanisms7,8, and similar aerodynamic valves seem to be present in crocodilians4,5,6. The anatomical and developmental similarities in the primary and secondary bronchi of birds and crocodilians suggest that these structures and airflow patterns may be homologous4,5,6,9. The origin of this pattern is at least as old as the split between crocodilians and birds, which occurred in the Triassic period10. Alternatively, this pattern of flow may be even older; this hypothesis can be tested by investigating patterns of airflow in members of the outgroup to birds and crocodilians, the Lepidosauromorpha (tuatara, lizards and snakes). Here we demonstrate region-specific unidirectional airflow in the lungs of the savannah monitor lizard (Varanus exanthematicus). The presence of unidirectional flow in the lungs of V. exanthematicus thus gives rise to two possible evolutionary scenarios: either unidirectional airflow evolved independently in archosaurs and monitor lizards, or these flow patterns are homologous in archosaurs and V. exanthematicus, having evolved only once in ancestral diapsids (the clade encompassing snakes, lizards, crocodilians and birds). If unidirectional airflow is plesiomorphic for Diapsida, this respiratory character can be reconstructed for extinct diapsids, and evolved in a small ectothermic tetrapod during the Palaeozoic era at least a hundred million years before the origin of birds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Maina, J. N. Development, structure, and function of a novel respiratory organ, the lung-air sac system of birds: to go where no other vertebrate has gone. Biol. Rev. Cambr. Phil. Soc. 81, 545–579 (2006)
Brackenbury, J. H. Lung-air-sac anatomy and respiratory pressures in the bird. J. Exp. Biol. 57, 543–550 (1972)
Maina, J. N. Spectacularly robust! Tensegrity principle explains the mechanical strength of the avian lung. Respir. Physiol. Neurobiol. 155, 1–10 (2007)
Farmer, C. G. The provenance of alveolar and parabronchial lungs: insights from paleoecology and the discovery of cardiogenic, unidirectional airflow in the American alligator (Alligator mississippiensis). Physiol. Biochem. Zool. 83, 561–575 (2010)
Farmer, C. G. & Sanders, K. Unidirectional airflow in the lungs of alligators. Science 327, 338–340 (2010)
Schachner, E. R., Hutchinson, J. R. & Farmer, C. G. Pulmonary anatomy in the Nile crocodile and the evolution of unidirectional airflow in Archosauria. PeerJ http://dx.doi.org/10.7717/peerj.60 (2013)
Butler, J. P., Banzett, R. B. & Fredberg, J. J. Inspiratory valving in avian bronchi: aerodynamic considerations. Respir. Physiol. 72, 241–255 (1988)
Hazelhoff, E. H. Structure and function of the lung of birds. Poult. Sci. 30, 3–10 (1951)
Sanders, R. K. & Farmer, C. G. The pulmonary anatomy of Alligator mississippiensis and its similarity to the avian respiratory system. Anat. Rec. 295, 699–714 (2012)
Nesbitt, S. J. The early evolution of archosaurs: relationships and the origin of major clades. Bull. Am. Mus. Nat. Hist. 352, 1–292 (2011)
Perry, S. F. in Biology of the Reptilia Vol. 19 (Morphology G) (eds Gans, C. & Gaunt, A. S. ) 1–92 (Society for the Study of Amphibians and Reptiles, 1998)
Conrad, J. L., Balcarcel, A. M. & Mehling, C. M. Earliest example of a giant monitor lizard (Varanus, Varanidae, Squamata). PLoS ONE 7, e41767 (2012)
Holmes, R. B., Murray, A. M., Attia, Y. S., Simons, E. L. & Chatrath, P. Oldest known Varanus (Squamata: Varanidae) from the Upper Eocene and Lower Oligocene of Egypt: support for an African origin of the genus. Palaeontology 53, 1099–1110 (2010)
Collar, D. C., Schulte, J. A., II & Losos, J. B. Evolution of extreme body size disparity in monitor lizards (Varanus). Evolution 65, 2664–2680 (2011)
Pianka, E. R. Evolution of body size: varanid lizards as a model system. Am. Nat. 146, 398–414 (1995)
Thompson, G. G. & Withers, P. C. Standard and maximal metabolic rates of goannas (Squamata: Varanidae). Physiol. Zool. 70, 307–323 (1997)
Owerkowicz, T., Farmer, C. G., Hicks, J. W. & Brainerd, E. L. Contribution of the gular pump to ventilation. Science 284, 1661–1663 (1999)
Becker, H.-O., Böhme, W. & Perry, S. F. Die Lungenmorphologie der Warane (Reptilia: Varanidae) und ihre systematisch-stammesgeschichtliche Bedeutung. Bonn. Zool. Beitr. 40, 27–56 (1989)
Burnell, A., Collins, S. & Young, B. A. The postpulmonary septum of Varanus salvator and its implication for Mosasaurian ventilation and physiology. Bull. Soc. Geol. Fr. 183, 159–169 (2012)
Kirschfeld, U. Eine Bauplananalyse der Waranlunge. Zool. Beitr. Neue Folge 16, 401–440 (1970)
Maina, J. N., Maloiy, G. M. O., Warui, C. N., Njogu, E. K. & Kokwaro, E. D. Scanning electron microscope study of the morphology of the reptilian lung: the savanna monitor lizard Varanus exanthematicus and the Pancake Tortoise Malacochersus tornieri. Anat. Rec. 224, 514–522 (1989)
Perry, S. F. & Duncker, H. R. Lung architecture, volume and static mechanics in five species of lizards. Respir. Physiol. 34, 61–81 (1978)
Wallach, V. in Biology of the Reptilia Vol. 19 (Morphology G) (eds Gans, C. & Gaunt, A. S. ) 93–295 (Society for the Study of Amphibians and Reptiles, 1998)
Milani, A. Beiträge zur Kenntniss der Reptilienlunge. Zool. Jahrb. 7, 545–592 (1894)
Milani, A. Beiträge zur Kenntnis der Reptilienlunge. II. Zool. Jahrb. 10, 93–156 (1897)
Milsom, W. K. & Vitalis, T. Z. Pulmonary mechanics and the work of breathing in the lizard, Gekko gecko. J. Exp. Biol. 113, 187–202 (1984)
Acknowledgements
We thank J. Dix (Reptile Rescue Service) for the donation of deceased varanid specimens, J. Bourke for assistance with Avizo, and D. Shafer for German translations. This work was supported by an American Association of Anatomists Postdoctoral Fellowship and an American Philosophical Society Franklin Research Grant to E.R.S., National Science Foundation grants to C.G.F. (IOS-1055080 and IOS-0818973) and a generous donation to the Farmer laboratory by S. Meyer.
Author information
Authors and Affiliations
Contributions
E.R.S. and R.L.C. conducted the in vivo surgeries. All authors collected data on excised lungs. E.R.S. acquired the CT scans and generated the three-dimensional digital models. C.G.F. and J.P.B. supervised and contributed ideas throughout the project. All authors contributed to the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related audio
41586_2014_BFnature12871_MOESM6_ESM.mp3
Some lizards breathe like birds, using a one-way system to get air through their bodies. Research Emma Schachner explains what that means for evolution.
Supplementary information
3D model of the skeletal and pulmonary anatomy of Varanus exanthematicus
A volume rendered three dimensional skeleton and segmented surface of the lungs and bronchial tree (left craniolateral view) of a female Varanus exanthematicus generated from a CT scan. The bronchus in which in vivo unidirectional flow was measured is indicated. Abbreviations: cb, cervical bronchus; L1-L10, lateral bronchi 1-10; M1-M11, medial bronchi 1-11. (MP4 29060 kb)
Unidirectional movement of fluid through regions of the lung in V. exanthematicus
Microsphere infused saline flowing from lateral bronchus 10 to lateral bronchus 9 in an excised right lung during manual ventilation (60 cc syringe). The microspheres can be seen moving from right to left (caudal to cranial) during inspiration and expiration. Abbreviations: L9, lateral bronchus 9; L10, lateral bronchus 10. (MP4 27392 kb)
Rights and permissions
About this article
Cite this article
Schachner, E., Cieri, R., Butler, J. et al. Unidirectional pulmonary airflow patterns in the savannah monitor lizard. Nature 506, 367–370 (2014). https://doi.org/10.1038/nature12871
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12871
This article is cited by
-
Body size, shape and ecology in tetrapods
Nature Communications (2022)
-
Unidirectional pulmonary airflow in vertebrates: a review of structure, function, and evolution
Journal of Comparative Physiology B (2016)
-
Why lizards may inherit the Earth
Nature (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.