Abstract
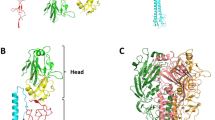
Of the 132 people known to have been infected with H7N9 influenza viruses in China, 37 died, and many were severely ill1. Infection seems to have involved contact with infected poultry2,3. We have examined the receptor-binding properties of this H7N9 virus and compared them with those of an avian H7N3 virus. We find that the human H7 virus has significantly higher affinity for α-2,6-linked sialic acid analogues (‘human receptor’) than avian H7 while retaining the strong binding to α-2,3-linked sialic acid analogues (‘avian receptor’) characteristic of avian viruses. The human H7 virus does not, therefore, have the preference for human versus avian receptors characteristic of pandemic viruses. X-ray crystallography of the receptor-binding protein, haemagglutinin (HA), in complex with receptor analogues indicates that both human and avian receptors adopt different conformations when bound to human H7 HA than they do when bound to avian H7 HA. Human receptor bound to human H7 HA exits the binding site in a different direction to that seen in complexes formed by HAs from pandemic viruses4,5 and from an aerosol-transmissible H5 mutant6. The human-receptor-binding properties of human H7 probably arise from the introduction of two bulky hydrophobic residues by the substitutions Gln226Leu and Gly186Val. The former is shared with the 1957 H2 and 1968 H3 pandemic viruses and with the aerosol-transmissible H5 mutant. We conclude that the human H7 virus has acquired some of the receptor-binding characteristics that are typical of pandemic viruses, but its retained preference for avian receptor may restrict its further evolution towards a virus that could transmit efficiently between humans, perhaps by binding to avian-receptor-rich mucins in the human respiratory tract7 rather than to cellular receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
World Health Organization. Number of confirmed human cases of avian influenza A(H7N9) reported to WHO Report 8 - data in WHO/HQ as of 30 May 2013, 15:45 GMT+1. http://www.who.int/influenza/human_animal_interface/influenza_h7n9/08_ReportWebH7N9Number.pdf (2013)
Chen, Y. et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet 381, 1916–1925 (2013)
Gao, R. et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 368, 1888–1897 (2013)
Eisen, M. B., Sabesan, S., Skehel, J. J. & Wiley, D. C. Binding of the influenza A virus to cell-surface receptors: structures of five hemagglutinin–sialyloligosaccharide complexes determined by X-ray crystallography. Virology 232, 19–31 (1997)
Liu, J. et al. Structures of receptor complexes formed by hemagglutinins from the Asian influenza pandemic of 1957. Proc. Natl Acad. Sci. USA 106, 17175–17180 (2009)
Xiong, X. et al. Receptor binding by a ferret-transmissible H5 avian influenza virus. Nature 497, 392–396 (2013)
Couceiro, J. N., Paulson, J. C. & Baum, L. G. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium; the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 29, 155–165 (1993)
Kageyama, T. et al. Genetic analysis of novel avian A(H7N9) influenza viruses isolated from patients in China, February to April 2013. Eurosurveillance 18, 20453 (2013)
Butt, K. M. et al. Human infection with an avian H9N2 influenza A virus in Hong Kong in 2003. J. Clin. Microbiol. 43, 5760–5767 (2005)
Lin, Y. P. et al. Avian-to-human transmission of H9N2 subtype influenza A viruses: relationship between H9N2 and H5N1 human isolates. Proc. Natl Acad. Sci. USA 97, 9654–9658 (2000)
Peiris, M. et al. Human infection with influenza H9N2. Lancet 354, 916–917 (1999)
Russell, R. J., Stevens, D. J., Haire, L. F., Gamblin, S. J. & Skehel, J. J. Avian and human receptor binding by hemagglutinins of influenza A viruses. Glycoconj. J. 23, 85–92 (2006)
Ha, Y., Stevens, D. J., Skehel, J. J. & Wiley, D. C. X-ray structures of H5 avian and H9 swine influenza virus hemagglutinins bound to avian and human receptor analogs. Proc. Natl Acad. Sci. USA 98, 11181–11186 (2001)
Gambaryan, A. S. et al. Receptor-binding profiles of H7 subtype influenza viruses in different host species. J. Virol. 86, 4370–4379 (2012)
Gamblin, S. J. et al. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science 303, 1838–1842 (2004)
Russell, R. J. et al. H1 and H7 influenza haemagglutinin structures extend a structural classification of haemagglutinin subtypes. Virology 325, 287–296 (2004)
Zhang, W. et al. An airborne transmissible avian influenza H5 hemagglutinin seen at the atomic level. Science http://dx.doi.org/10.1126/science.1236787 (2 May 2013)
Baigent, S. J. & McCauley, J. W. Influenza type A in humans, mammals and birds: determinants of virus virulence, host-range and interspecies transmission. BioEssays 25, 657–671 (2003)
Wagner, R., Matrosovich, M. & Klenk, H. D. Functional balance between haemagglutinin and neuraminidase in influenza virus infections. Rev. Med. Virol. 12, 159–166 (2002)
Garten, W. & Klenk, H. D. Understanding influenza virus pathogenicity. Trends Microbiol. 7, 99–100 (1999)
Skehel, J. J. & Wiley, D. C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69, 531–569 (2000)
Steinhauer, D. A. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 258, 1–20 (1999)
Daniels, R. S. et al. Fusion mutants of the influenza virus hemagglutinin glycoprotein. Cell 40, 431–439 (1985)
Ruigrok, R. W. et al. Conformational changes in the hemagglutinin of influenza virus which accompany heat-induced fusion of virus with liposomes. Virology 155, 484–497 (1986)
Imai, M. et al. Experimental adaptation of an influenza H5 HA confers respiratory droplet transmission to a reassortant H5 HA/H1N1 virus in ferrets. Nature 486, 420–428 (2012)
Lin, Y. P. et al. Evolution of the receptor binding properties of the influenza A(H3N2) haemagglutinin. Proc. Natl Acad. Sci. USA 109, 21474–21479 (2012)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Collaborative Computational Project, number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Skehel, J. J. & Schild, G. C. The polypeptide composition of influenza A viruses. Virology 44, 396–408 (1971)
Acknowledgements
We acknowledge Y. Shu for sharing information and samples on recent H7N9 virus infections. We are grateful to staff at the Diamond Light Source Synchrotron for assistance and beamline access under proposal 7707 and the staff of the NIMR Large Scale laboratory. This work was funded by the Medical Research Council through programmes U117584222, U117570592, U117585868 and U117512723.
Author information
Authors and Affiliations
Contributions
X.X., S.R.M., L.F.H., S.A.W., R.S.D., M.S.B., J.W.M., P.J.C., P.A.W., J.J.S. and S.J.G. all performed experiments and contributed to the writing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1–6 and Supplementary Tables 1–3. (PDF 2218 kb)
Rights and permissions
About this article
Cite this article
Xiong, X., Martin, S., Haire, L. et al. Receptor binding by an H7N9 influenza virus from humans. Nature 499, 496–499 (2013). https://doi.org/10.1038/nature12372
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12372
This article is cited by
-
Alternative antiviral approaches to combat influenza A virus
Virus Genes (2023)
-
Avian influenza A (H7N9) virus: from low pathogenic to highly pathogenic
Frontiers of Medicine (2021)
-
One health insights to prevent the next HxNy viral outbreak: learning from the epidemiology of H7N9
BMC Infectious Diseases (2019)
-
Host and viral determinants of influenza A virus species specificity
Nature Reviews Microbiology (2019)
-
Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors
Nature Structural & Molecular Biology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.