Abstract
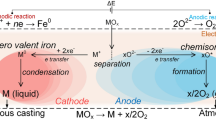
Molten oxide electrolysis (MOE) is an electrometallurgical technique that enables the direct production of metal in the liquid state from oxide feedstock1,2, and compared with traditional methods of extractive metallurgy offers both a substantial simplification of the process and a significant reduction in energy consumption3. MOE is also considered a promising route for mitigation of CO2 emissions in steelmaking3,4,5, production of metals free of carbon6, and generation of oxygen for extra-terrestrial exploration7,8. Until now, MOE has been demonstrated using anode materials that are consumable (graphite for use with ferro-alloys and titanium6,9) or unaffordable for terrestrial applications (iridium for use with iron10,11). To enable metal production without process carbon, MOE requires an anode material that resists depletion while sustaining oxygen evolution. The challenges for iron production are threefold. First, the process temperature is in excess of 1,538 degrees Celsius (ref. 10). Second, under anodic polarization most metals inevitably corrode in such conditions11,12,13. Third, iron oxide undergoes spontaneous reduction on contact with most refractory metals14 and even carbon. Here we show that anodes comprising chromium-based alloys exhibit limited consumption during iron extraction and oxygen evolution by MOE. The anode stability is due to the formation of an electronically conductive solid solution of chromium(iii) and aluminium oxides in the corundum structure. These findings make practicable larger-scale evaluation of MOE for the production of steel, and potentially provide a key material component enabling mitigation of greenhouse-gas emissions while producing metal of superior metallurgical quality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Aiken, R. H. Process of making iron from the ore. US patent 816. 142 (1906)
Esin, O. A. Electrochemistry of molten oxides. Zh. Fiz. Khim. 30, 3–19 (1956)
Birat, J.-P., Lorrain, J.-P. & de Lassat, Y. The “CO2 tool”: CO2 emissions and energy consumption of existing and breakthrough steelmaking routes. Rev. Métal. 106, 325–336 (2009)
International Energy Agency. Energy Technology Perspective 179–180 (OECD/IEA, Paris, 2010); available at http://www.iea.org/publications/freepublications/publication/etp2010.pdf
Halper, M. Stainless steel. Time 177, 7–9 (2011)
Winand, R., Fontana, A., Segers, L., Hannaert, P. & Lacave, J. in Int. Symp. Molten Salt Electrolysis in Metal Production 42–50 (Institute of Mining and Metallurgy, London, 1977)
Oppenheim, M. J. Electrolysis of molten basalt. Mineral. Mag. 36, 1104–1122 (1968)
Sanderson, F. How to breathe on the moon. Nature http://dx.doi.org/10.1038/news.2009.803 (published online, 10 August 2009)
Sadoway, D. R. Electrochemical processing of refractory metals. J. Met. 43, 15–19 (1991)
Wang, D., Gmitter, A. J. & Sadoway, D. R. Production of oxygen gas and liquid metal by electrochemical decomposition of molten iron oxide. J. Electrochem. Soc. 158, E51–E54 (2011)
Kim, H., Paramore, J., Allanore, A. & Sadoway, D. R. Electrolysis of molten iron oxide with an iridium anode: the role of electrolyte basicity. J. Electrochem. Soc. 158, E101–E105 (2011)
Sadoway, D. R. Inert anodes for the Hall-Héroult cell: the ultimate materials challenge. J. Met. 53, 34–35 (2001)
Di Martino, J., Rapin, C., Berthod, P., Podor, R. & Steinmetz, P. Corrosion of metals and alloys in molten glasses. Part 2: nickel and cobalt high chromium superalloys behaviour and protection. Corros. Sci. 46, 1865–1881 (2004)
Carton, A., Rapin, C., Podor, R. & Berthod, P. Corrosion of chromium in glass melts. J. Electrochem. Soc. 153, B121–B127 (2006)
Takahashi, N. T. et al. Cr diffusion in α-Al2O3: secondary ion mass spectroscopy and first-principles study. Phys. Rev. B 82, 174302 (2010)
Egerton, T. A., Stone, F. S. & Vickerman, J. C. α-Cr2O3-Al2O3 solid solutions 1. The formation and stability of adsorbed oxygen. J. Catal. 33, 299–306 (1974)
Nguyen, T. & de Nora, V. in Light Metals (ed. Galloway, T. J. ) 385–390 (The Minerals, Metals and Materials Society, 2006)
MacDonald, D. The history of the point defect model for the passive state: a brief review of film growth aspects. Electrochim. Acta 56, 1761–1772 (2011)
Acknowledgements
The financial support of the American Iron and Steel Institute is acknowledged; we thank H. Kim and J. Paramore for assistance with the experimental set-up and for discussions.
Author information
Authors and Affiliations
Contributions
A.A. conceived the idea and designed the study based on principles enunciated by D.R.S. A.A. and Y.L. performed the experiments, the analysis of the results, and wrote the original draft of the paper. D.R.S. edited the original manuscript and revised it for submission. All authors discussed the results and commented on the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains a description of the Supplementary Video, Supplementary Figures 1-2 showing the influence of the alloy composition and electrolysis duration on the material stability, a section A dedicated to the investigation of the chemical stability of chromium oxide (+III) in the investigated electrolyte, and a section B in which chromium and chromium-iron alloys oxidation kinetics at high temperature are investigated. (PDF 30019 kb)
Observation of the gas evolution obtained with a cell operating with the novel anode material at 1565°C
Video recorded from the top of the tube furnace, it shows from above the entire pool of molten oxide contained in the crucible. The anode is located at the center and is noticeable thanks to its brightness. The electrolyte convection inherited from the gas evolution brings hotter electrolyte toward the cell surface and allows to visualize the gas evolution thanks to the corresponding contrast difference. (MP4 28670 kb)
Rights and permissions
About this article
Cite this article
Allanore, A., Yin, L. & Sadoway, D. A new anode material for oxygen evolution in molten oxide electrolysis. Nature 497, 353–356 (2013). https://doi.org/10.1038/nature12134
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature12134
This article is cited by
-
In-Situ Precipitated Needle Like Nanocrystalline β-Ti Reinforced Porous Titanium Alloy via Molten Salt Electrolysis
Metals and Materials International (2024)
-
An Electrolysis-Distillation Approach for Producing Potassium Metal
Metallurgical and Materials Transactions B (2024)
-
An iron-base oxygen-evolution electrode for high-temperature electrolyzers
Nature Communications (2023)
-
Oxygen evolution behavior of La1−xSrxFeO3−δ electrodes in LiCl–KCl melt
Journal of Applied Electrochemistry (2023)
-
Preliminary Economic Analysis of Red Mud Valorization via Colloidal Aqueous Electrolytic Reduction in a Modern Electricity Infrastructure
Journal of Sustainable Metallurgy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.