Abstract
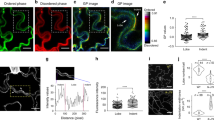
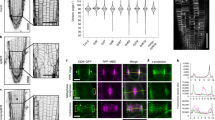
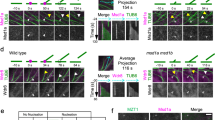
Recent evidence indicates a correlation between orientation of the plant cortical microtubule cytoskeleton and localization of polar cargoes1. However, the molecules and mechanisms that create this correlation have remained unknown. Here we show that, in Arabidopsis thaliana, the microtubule orientation regulators CLASP2,3 and MAP65 (refs 3, 4) control the abundance of polarity regulator PINOID kinase5,6 at the plasma membrane. By localized upregulation of clathrin-dependent endocytosis7 at cortical microtubule- and clathrin-rich domains orthogonal to the axis of polarity, PINOID accelerates the removal of auxin transporter PIN proteins8,9,10 from those sites. This mechanism links directional microtubule organization to the polar localization of auxin transporter PIN proteins, and clarifies how microtubule-enriched cell sides are kept distinct from polar delivery domains. Our results identify the molecular machinery that connects microtubule organization to the regulation of the axis of PIN polarization.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Heisler, M. G. et al. Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 8, e1000516 (2010)
Ambrose, C., Allard, J. F., Cytrynbaum, E. N. & Wasteneys, G. O. A CLASP-modulated cell edge barrier mechanism drives cell-wide cortical microtubule organization in Arabidopsis . Nature Commun. 2, 430 (2011)
Dhonukshe, P. et al. A PLETHORA-auxin transcription module controls cell division plane rotation through MAP65 and CLASP. Cell 149, 383–396 (2012)
Smertenko, A. et al. A new class of microtubule-associated proteins in plants. Nature Cell Biol. 2, 750–753 (2000)
Friml, J. et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306, 862–865 (2004)
Dhonukshe, P. et al. Plasma membrane-bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN recycling. Development 137, 3245–3255 (2010)
Dhonukshe, P. et al. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis . Curr. Biol. 17, 520–527 (2007)
Blilou, I. et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44 (2005)
Wisniewska, J. et al. Polar PIN localization directs auxin flow in plants. Science 312, 883 (2006)
Dhonukshe, P. et al. Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature 456, 962–966 (2008)
Li, R. & Gundersen, G. G. Beyond polymer polarity: how the cytoskeleton builds a polarized cell. Nature Rev. Mol. Cell Biol. 9, 860–873 (2008)
Fu, Y., Gu, Y., Zheng, Z., Wasteneys, G. & Yang, Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 120, 687–700 (2005)
Nagawa, S. et al. ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS Biol. 10, e1001299 (2012)
Xu, T. et al. Cell surface- and rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis . Cell 143, 99–110 (2010)
Ambrose, J. C., Shoji, T., Kotzer, A. M., Pighin, J. A. & Wasteneys, G. O. The Arabidopsis CLASP gene encodes a microtubule-associated protein involved in cell expansion and division. Plant Cell 19, 2763–2775 (2007)
Geldner, N., Friml, J., Stierhof, Y. D., Jurgens, G. & Palme, K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413, 425–428 (2001)
Dhonukshe, P. et al. Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev. Cell 10, 137–150 (2006)
Dharmasiri, N. et al. Plant development is regulated by a family of auxin receptor F box proteins. Dev. Cell 9, 109–119 (2005)
Hayashi, K. et al. Small-molecule agonists and antagonists of F-box protein-substrate interactions in auxin perception and signaling. Proc. Natl Acad. Sci. USA 105, 5632–5637 (2008)
Dhonukshe, P., Mathur, J., Hulskamp, M. & Gadella, T. W., Jr Microtubule plus-ends reveal essential links between intracellular polarization and localized modulation of endocytosis during division-plane establishment in plant cells. BMC Biol. 3, 11 (2005)
Karahara, I. et al. The preprophase band is a localized center of clathrin-mediated endocytosis in late prophase cells of the onion cotyledon epidermis. Plant J. 57, 819–831 (2009)
Di Laurenzio, L. et al. The SCARECROW gene regulates an asymmetric cell division that is essential for generating the radial organization of the Arabidopsis root. Cell 86, 423–433 (1996)
Stepanova, A. N. et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191 (2008)
Sasabe, M., Kosetsu, K., Hidaka, M., Murase, A. & Machida, Y. Arabidopsis thaliana MAP65-1 and MAP65-2 function redundantly with MAP65-3/PLEIADE in cytokinesis downstream of MPK4. Plant Signal. Behav. 6, 743–747 (2011)
Heisler, M. G. et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 15, 1899–1911 (2005)
Xu, J. & Scheres, B. Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17, 525–536 (2005)
Benková, E. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 (2003)
Michniewicz, M. et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130, 1044–1056 (2007)
Galinha, C. et al. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature 449, 1053–1057 (2007)
Konopka, C. A., Backues, S. K. & Bednarek, S. Y. Dynamics of Arabidopsis dynamin-related protein 1C and a clathrin light chain at the plasma membrane. Plant Cell 20, 1363–1380 (2008)
Kotogány, E., Dudits, D., Horvath, G. V. & Ayaydin, F. A rapid and robust assay for detection of S-phase cell cycle progression in plant cells and tissues by using ethynyl deoxyuridine. Plant Methods 6, 5 (2010)
Sauer, M., Paciorek, T., Benkova, E. & Friml, J. Immunocytochemical techniques for whole-mount in situ protein localization in plants. Nature Protocols 1, 98–103 (2006)
Abas, L. et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nature Cell Biol. 8, 249–256 (2006)
Kim, Y. W. et al. Arabidopsis dynamin-like 2 that binds specifically to phosphatidylinositol 4-phosphate assembles into a high-molecular weight complex in vivo and in vitro. Plant Physiol. 127, 1243–1255 (2001)
Acknowledgements
We thank M. Estelle, G. Wasteneys, K. Hayashi, C. Luschnig, I. Hwang, M. Sasabe and M. Heisler for sharing published material. We thank F. Kindt and R. Leito for photography and image processing, and the Akhmanova laboratory for the western blotting facility. This work was supported by the Utrecht University’s Starting Independent Investigator, the Netherlands Organisation for Scientific Research VIDI and European Research Council Starting Investigator grants to P.D., and the European Research Council Advanced Investigator grant, the Netherlands Organisation for Scientific Research Spinoza and Netherlands Proteomics Centre grants to B.S. K.K. was recruited from grants of P.D., and H.Z. was supported by grants of B.S.
Author information
Authors and Affiliations
Contributions
P.D. conceptualized the project, designed the experiments and directed the work; B.S. contributed to the experimental design; K.K. and P.D. performed experiments and analysed data; H.Z. contributed to western analysis; P.D., K.K. and B.S. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1 and Supplementary Figures 1-10. (PDF 9120 kb)
Rights and permissions
About this article
Cite this article
Kakar, K., Zhang, H., Scheres, B. et al. CLASP-mediated cortical microtubule organization guides PIN polarization axis. Nature 495, 529–533 (2013). https://doi.org/10.1038/nature11980
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11980
This article is cited by
-
Functions of long non-coding RNAs in plants: a riddle to explore
The Nucleus (2018)
-
Correction: Retraction: CLASP-mediated cortical microtubule organization guides PIN polarization axis
Nature (2014)
-
The far side of auxin signaling: fundamental cellular activities and their contribution to a defined growth response in plants
Protoplasma (2014)
-
Arabidopsis SABRE and CLASP interact to stabilize cell division plane orientation and planar polarity
Nature Communications (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.