Abstract
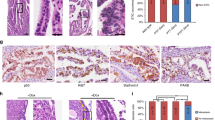
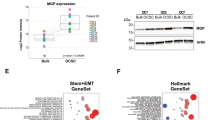
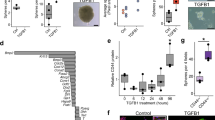
Epithelial ovarian cancer (EOC) is the fifth leading cause of cancer deaths among women in the United States, but its pathogenesis is poorly understood1,2,3. Some epithelial cancers are known to occur in transitional zones between two types of epithelium, whereas others have been shown to originate in epithelial tissue stem cells4,5,6. The stem cell niche of the ovarian surface epithelium (OSE), which is ruptured and regenerates during ovulation, has not yet been defined unequivocally. Here we identify the hilum region of the mouse ovary, the transitional (or junction) area between the OSE, mesothelium and tubal (oviductal) epithelium, as a previously unrecognized stem cell niche of the OSE. We find that cells of the hilum OSE are cycling slowly and express stem and/or progenitor cell markers ALDH1, LGR5, LEF1, CD133 and CK6B. These cells display long-term stem cell properties ex vivo and in vivo, as shown by our serial sphere generation and long-term lineage-tracing assays. Importantly, the hilum cells show increased transformation potential after inactivation of tumour suppressor genes Trp53 and Rb1, whose pathways are altered frequently in the most aggressive and common type of human EOC, high-grade serous adenocarcinoma7,8. Our study supports experimentally the idea that susceptibility of transitional zones to malignant transformation may be explained by the presence of stem cell niches in those areas. Identification of a stem cell niche for the OSE may have important implications for understanding EOC pathogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Siegel, R., Naishadham, D. & Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 62, 10–29 (2012)
Lengyel, E. Ovarian cancer development and metastasis. Am. J. Pathol. 177, 1053–1064 (2010)
Bowtell, D. D. The genesis and evolution of high-grade serous ovarian cancer. Nature Rev. Cancer 10, 803–808 (2010)
Visvader, J. E. Cells of origin in cancer. Nature 469, 314–322 (2011)
Auersperg, N. The origin of ovarian carcinomas: a unifying hypothesis. Int. J. Gynecol. Pathol. 30, 12–21 (2011)
Medema, J. P. & Vermeulen, L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature 474, 318–326 (2011)
The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011)
Ahmed, A. A. et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J. Pathol. 221, 49–56 (2010)
Kurman, R. J. & Shih Ie, M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am. J. Surg. Pathol. 34, 433–443 (2010)
Dubeau, L. The cell of origin of ovarian epithelial tumours. Lancet Oncol. 9, 1191–1197 (2008)
Auersperg, N., Woo, M. M. & Gilks, C. B. The origin of ovarian carcinomas: a developmental view. Gynecol. Oncol. 110, 452–454 (2008)
Seidman, J. D., Yemelyanova, A., Zaino, R. J. & Kurman, R. J. The fallopian tube-peritoneal junction: a potential site of carcinogenesis. Int. J. Gynecol. Pathol. 30, 4–11 (2011)
Szotek, P. P. et al. Normal ovarian surface epithelial label-retaining cells exhibit stem/progenitor cell characteristics. Proc. Natl Acad. Sci. USA 105, 12469–12473 (2008)
Blanpain, C., Horsley, V. & Fuchs, E. Epithelial stem cells: turning over new leaves. Cell 128, 445–458 (2007)
Simons, B. D. & Clevers, H. Strategies for homeostatic stem cell self-renewal in adult tissues. Cell 145, 851–862 (2011)
Ginestier, C. et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1, 555–567 (2007)
Storms, R. W. et al. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc. Natl Acad. Sci. USA 96, 9118–9123 (1999)
Corti, S. et al. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells 24, 975–985 (2006)
Burger, P. E. et al. High aldehyde dehydrogenase activity: a novel functional marker of murine prostate stem/progenitor cells. Stem Cells 27, 2220–2228 (2009)
Deng, S. et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS ONE 5, e10277 (2010)
Bullough, W. S. Oogenesis and its relation to the oestrous cycle in the adult mouse. J. Endocrinol. 3, 141–149 (1942)
Ji, Q. et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE 4, e6816 (2009)
Liu, C. et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nature Med. 17, 211–215 (2011)
Choi, Y. J. et al. miR-34 miRNAs provide a barrier for somatic cell reprogramming. Nature Cell Biol. 13, 1353–1360 (2011)
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007)
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature Neurosci. 13, 133–140 (2010)
Flesken-Nikitin, A., Choi, K. C., Eng, J. P., Shmidt, E. N. & Nikitin, A. Y. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 63, 3459–3463 (2003)
Szabova, L. et al. Perturbation of Rb, p53, and Brca1 or Brca2 cooperate in inducing metastatic serous epithelial ovarian cancer. Cancer Res. 72, 4141–4153 (2012)
Pellegrini, G. et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J. Cell Biol. 145, 769–782 (1999)
Barker, N. et al. Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 (2010)
Corney, D. C., Flesken-Nikitin, A., Godwin, A. K., Wang, W. & Nikitin, A. Y. MicroRNA-34b and microRNA-34c are targets of p53 and cooperate in control of cell proliferation and adhesion-independent growth. Cancer Res. 67, 8433–8438 (2007)
Hwang, C. I. et al. Wild-type p53 controls cell motility and invasion by dual regulation of MET expression. Proc. Natl Acad. Sci. USA 108, 14240–14245 (2011)
Russo, J. E., Hauguitz, D. & Hilton, J. Inhibition of mouse cytosolic aldehyde dehydrogenase by 4-(diethylamino)benzaldehyde. Biochem. Pharmacol. 37, 1639–1642 (1988)
Lawson, D. A., Xin, L., Lukacs, R. U., Cheng, D. & Witte, O. N. Isolation and functional characterization of murine prostate stem cells. Proc. Natl Acad. Sci. USA 104, 181–186 (2007)
Xin, L., Lukacs, R. U., Lawson, D. A., Cheng, D. & Witte, O. N. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells 25, 2760–2769 (2007)
Singec, I. et al. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nature Methods 3, 801–806 (2006)
Zhou, Z. et al. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 66, 7889–7898 (2006)
Choi, J., Curtis, S. J., Roy, D. M., Flesken-Nikitin, A. & Nikitin, A. Y. Local mesenchymal stem/progenitor cells are a preferential target for initiation of adult soft tissue sarcomas associated with p53 and Rb deficiency. Am. J. Pathol. 177, 2645–2658 (2010)
Nikitin, A. Y. & Lee, W. H. Early loss of the retinoblastoma gene is associated with impaired growth inhibitory innervation during melanotroph carcinogenesis in Rb+/− mice. Genes Dev. 10, 1870–1879 (1996)
Debacq-Chainiaux, F., Erusalimsky, J. D., Campisi, J. & Toussaint, O. Protocols to detect senescence-associated beta-galactosidase (SA-βgal) activity, a biomarker of senescent cells in culture and in vivo. Nature Protocols 4, 1798–1806 (2009)
Matoso, A., Zhou, Z., Hayama, R., Flesken-Nikitin, A. & Nikitin, A. Y. Cell lineage-specific interactions between Men1 and Rb in neuroendocrine neoplasia. Carcinogenesis 29, 620–628 (2008)
Zhou, Z., Flesken-Nikitin, A. & Nikitin, A. Y. Prostate cancer associated with p53 and Rb deficiency arises from the stem/progenitor cell-enriched proximal region of prostatic ducts. Cancer Res. 67, 5683–5690 (2007)
Zhou, Z. et al. Suppression of melanotroph carcinogenesis leads to accelerated progression of pituitary anterior lobe tumors and medullary thyroid carcinomas in Rb+/− mice. Cancer Res. 65, 787–796 (2005)
Rugh, R. In The Mouse: Its Reproduction and Development 7–43 (Oxford Univ. Press, 1990)
Vermot, J., Fraulob, V., Dolle, P. & Niederreither, K. Expression of enzymes synthesizing (aldehyde dehydrogenase 1 and reinaldehyde dehydrogenase 2) and metabolizaing (Cyp26) retinoic acid in the mouse female reproductive system. Endocrinology 141, 3638–3645 (2000)
Acknowledgements
We would like to thank T. Tumbar for critical reading of this manuscript, J. Choi for help with immunostainings, L. Sayam (NYSTEM supported FACS Core) for help with FACS experiments and F. M. Vermeylen (Cornell Statistical Consulting Unit) for help with statistical analysis. This work was supported by grants from the US National Institutes of Health (NIH) and National Cancer Institute (NCI) (CA096823 and CA112354), NYSTEM (C023050 and N11G-160) and the Marsha Rivkin Center for Ovarian Cancer Research to A.Yu.N.; the NIH National Institute of Mental Health (MH092928), NIH National Institute on Aging (AG040209), NYSTEM (C024323) and Russian Ministry of Education and Science to G.E.; and postdoctoral fellowships to A.F.-N. (NIH NICHD T32HD052471) and C-.I.H. (Cornell Comparative Cancer Biology Training Program).
Author information
Authors and Affiliations
Contributions
Author Contributions A.F.-N. and A.Yu.N. designed the study, interpreted data and wrote the manuscript. A.F.-N., C.-I.H., C.-Y.C., T.V.M. and G.E. performed experiments and analysed data. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-21 and Supplementary Tables 1-6. (PDF 5624 kb)
Rights and permissions
About this article
Cite this article
Flesken-Nikitin, A., Hwang, CI., Cheng, CY. et al. Ovarian surface epithelium at the junction area contains a cancer-prone stem cell niche. Nature 495, 241–245 (2013). https://doi.org/10.1038/nature11979
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11979
This article is cited by
-
Harnessing preclinical models for the interrogation of ovarian cancer
Journal of Experimental & Clinical Cancer Research (2022)
-
Differential epithelial and stromal LGR5 expression in ovarian carcinogenesis
Scientific Reports (2022)
-
Regulation of stem cell fate by HSPGs: implication in hair follicle cycling
npj Regenerative Medicine (2022)
-
A rare ovarian hilus cell tumour accompanying bilateral serous cystadenomas: report of a case
Journal of Ovarian Research (2021)
-
Transcriptional heterogeneity of stemness phenotypes in the ovarian epithelium
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.