Abstract
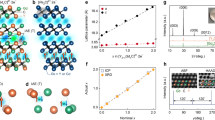
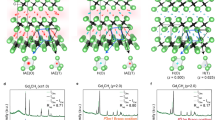
Recent studies suggest that electrides—ionic crystals in which electrons serve as anions—are not exceptional materials but rather a generalized form, particularly under high pressure1,2,3. The topology of the cavities confining anionic electrons determines their physical properties4. At present, reported confining sites consist only of zero-dimensional cavities or weakly linked channels4. Here we report a layered-structure electride of dicalcium nitride, Ca2N, which possesses two-dimensionally confined anionic electrons whose concentration agrees well with that for the chemical formula of [Ca2N]+·e−. Two-dimensional transport characteristics are demonstrated by a high electron mobility (520 cm2 V−1 s−1) and long mean scattering time (0.6 picoseconds) with a mean free path of 0.12 micrometres. The quadratic temperature dependence of the resistivity up to 120 Kelvin indicates the presence of an electron–electron interaction. A striking anisotropic magnetoresistance behaviour with respect to the direction of magnetic field (negative for the field perpendicular to the conducting plane and positive for the field parallel to it) is observed, confirming diffusive two-dimensional transport in dense electron layers. Additionally, band calculations support confinement of anionic electrons within the interlayer space, and photoemission measurements confirm anisotropic low work functions of 3.5 and 2.6 electronvolts, revealing the loosely bound nature of the anionic electrons. We conclude that Ca2N is a two-dimensional electride in terms of [Ca2N]+·e−.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Pickard, C. J. & Needs, R. J. Dense low-coordination phase of lithium. Phys. Rev. Lett. 102, 146401 (2009)
Ma, Y. et al. Transparent dense sodium. Nature 458, 182–185 (2009)
Pickard, C. J. & Needs, R. J. Aluminium at terapascal pressures. Nature Mater. 9, 624–627 (2010)
Dye, J. L. Electrides: early examples of quantum confinement. Acc. Chem. Res. 42, 1564–1572 (2009)
Kim, S. W., Shimoyama, T. & Hosono, H. Solvated electrons in high-temperature melts and glasses of the room-temperature stable electride [Ca24Al28O64]4+·4e− . Science 333, 71–74 (2011)
Toda, Y. et al. Field emission of electron anions clathrated in subnanometer-sized cages of [Ca24Al28O64]4+(4e-). Adv. Mater. 16, 685–689 (2004)
Ando, T., Fowler, A. B. & Stern, F. Electronic properties of two-dimensional systems. Rev. Mod. Phys. 54, 437–672 (1982)
Ohtomo, A. & Hwang, H. Y. A high mobility electron gas at the LaAlO3/SrTiO3 heterointerface. Nature 427, 423–426 (2004)
Gregory, D. H., Bowman, A., Baker, C. F. & Weston, D. P. Dicalcium nitride, Ca2N-a 2D “excess electron” compound; synthetic routes and crystal chemistry. J. Mater. Chem. 10, 1635–1641 (2000)
Reckeweg, O. & DiSalvo, F. J. Alkaline earth metal nitride compounds with the composition M2NX (M = Ca, Sr, Ba; X = □, H, Cl or Br). Solid State Sci. 4, 575–584 (2002)
Novoselov, K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004)
Sundar, V. C. et al. Elastomeric transistor stamps: reversible probing of charge transport in organic crystals. Science 303, 1644–1646 (2004)
Kittel, C. Introduction to Solid State Physics 8th edn (John Wiley & Sons, 2005)
Kasap, S. O. Principles of Electronic Materials and Devices 2nd edn (McGraw-Hill, 2002)
Matsuishi, S. et al. Localized and delocalized electrons in room-temperature stable electride [Ca24Al28O64]4+(O2−)2-x(e−)2x: analysis of optical reflectance spectra. J. Phys. Chem. C 112, 4753–4760 (2008)
Fox, M. Optical Properties of Solids (Oxford Univ. Press, 2001)
Oslen, J. L. Electron Transport in Metals (Interscience, 1962)
Piraux, L. Weak localization and coulomb interaction in graphite intercalation compounds and related materials. J. Mater. Res. 5, 1285–1298 (1990)
Li, Z., Yang, J., Hou, J. G. & Zhu, Q. Is mayenite without clathrated oxygen an inorganic electride? Angew. Chem. Int. Edn 43, 6479–6482 (2004)
Rourke, P. M. C. & Julian, S. R. Numerical extraction of de Haas-van Alphen frequencies from calculated band energies. Comput. Phys. Commun. 183, 324–332 (2012)
Steinbrenner, U., Adler, P., Hölle, W. & Simon, A. Electronic structure and chemical bonding in alkaline earth metal subnitrides: photoemission studies and band structure calculations. J. Phys. Chem. Solids 59, 1527–1536 (1998)
Demuth, J. E., Thompson, W. J., DiNardo, N. J. & Imbihl, R. Photoemission-based photovoltage probe of semiconductor surface and interface electronic structure. Phys. Rev. Lett. 56, 1408–1411 (1986)
Toda, Y. et al. Work function of a room-temperature, stable electride [Ca24Al28O64]4+(e-)4 . Adv. Mater. 19, 3564–3569 (2007)
Uijttewaal, M. A. de Wijs, G. A. & de Groot, R. A. Low work function of the (1000) Ca2N surface. J. Appl. Phys. 96, 1751–1753 (2004)
Tasker, P. W. The stability of ionic crystal surfaces. J. Phys. C 12, 4977–4984 (1979)
Grepstad, J. K., Gartland, P. O. & Slagsvold, B. J. Anisotropic work function of clean and smooth low-index faces of aluminium. Surf. Sci. 57, 348–362 (1976)
Kitano, M. et al. Ammonia synthesis using a stable electride as an electron donor and reversible hydrogen store. Nature Chem. 4, 934–940 (2012)
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996)
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994)
Kresse, G. & Furthmüller, J. Efficient iterative scheme for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996)
Blaha, P., Schwarz, K., Madsen, G. K. H., Kvasnicka, D. & Luitz, J. An Augmented Plane and Local Orbitals Program for Calculating Crystal Properties (ed. Schwarz, K ) (Technical University of Wien, 2001)
Acknowledgements
This work was supported by the Funding Program for World-Leading Innovative R&D on Science and Technology (FIRST), JSPS, and the Element Strategy Initiative Project, MEXT, Japan.
Author information
Authors and Affiliations
Contributions
H.H. conceived and S.W.K. initiated the study. K.L. and S.W.K. synthesized the samples and measured electron transport properties. Y.T. carried out the UPS measurement. S.M. performed DFT calculations. K.L., S.W.K. and H.H. co-wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data 1-6, Supplementary Figures 1-2, Supplementary Tables 1-3 and additional references. (PDF 384 kb)
Rights and permissions
About this article
Cite this article
Lee, K., Kim, S., Toda, Y. et al. Dicalcium nitride as a two-dimensional electride with an anionic electron layer. Nature 494, 336–340 (2013). https://doi.org/10.1038/nature11812
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11812
This article is cited by
-
Spectroscopic signature of obstructed surface states in SrIn2P2
Nature Communications (2023)
-
Critical role of hydrogen for superconductivity in nickelates
Nature (2023)
-
Magnetic quasi-atomic electrons driven reversible structural and magnetic transitions between electride and its hydrides
Nature Communications (2023)
-
Diversity of platinum-sites at platinum/fullerene interface accelerates alkaline hydrogen evolution
Nature Communications (2023)
-
Quantum electron liquid and its possible phase transition
Nature Materials (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.