Abstract
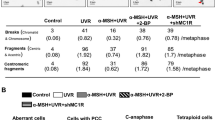
People with pale skin, red hair, freckles and an inability to tan—the ‘red hair/fair skin’ phenotype—are at highest risk of developing melanoma, compared to all other pigmentation types1. Genetically, this phenotype is frequently the product of inactivating polymorphisms in the melanocortin 1 receptor (MC1R) gene. MC1R encodes a cyclic AMP-stimulating G-protein-coupled receptor that controls pigment production. Minimal receptor activity, as in red hair/fair skin polymorphisms, produces the red/yellow pheomelanin pigment, whereas increasing MC1R activity stimulates the production of black/brown eumelanin2. Pheomelanin has weak shielding capacity against ultraviolet radiation relative to eumelanin, and has been shown to amplify ultraviolet-A-induced reactive oxygen species3,4,5. Several observations, however, complicate the assumption that melanoma risk is completely ultraviolet-radiation-dependent. For example, unlike non-melanoma skin cancers, melanoma is not restricted to sun-exposed skin and ultraviolet radiation signature mutations are infrequently oncogenic drivers6. Although linkage of melanoma risk to ultraviolet radiation exposure is beyond doubt, ultraviolet-radiation-independent events are likely to have a significant role1,7. Here we introduce a conditional, melanocyte-targeted allele of the most common melanoma oncoprotein, BRAFV600E, into mice carrying an inactivating mutation in the Mc1r gene (these mice have a phenotype analogous to red hair/fair skin humans). We observed a high incidence of invasive melanomas without providing additional gene aberrations or ultraviolet radiation exposure. To investigate the mechanism of ultraviolet-radiation-independent carcinogenesis, we introduced an albino allele, which ablates all pigment production on the Mc1re/e background. Selective absence of pheomelanin synthesis was protective against melanoma development. In addition, normal Mc1re/e mouse skin was found to have significantly greater oxidative DNA and lipid damage than albino-Mc1re/e mouse skin. These data suggest that the pheomelanin pigment pathway produces ultraviolet-radiation-independent carcinogenic contributions to melanomagenesis by a mechanism of oxidative damage. Although protection from ultraviolet radiation remains important, additional strategies may be required for optimal melanoma prevention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rhodes, A. R., Weinstock, M. A., Fitzpatrick, T. B., Mihm, M. C. J. & Sober, A. J. Risk factors for cutaneous melanoma. A practical method of recognizing predisposed individuals. J. Am. Med. Assoc. 258, 3146–3154 (1987)
Valverde, P., Healy, E., Jackson, I., Rees, J. L. & Thody, A. J. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nature Genet. 11, 328–330 (1995)
Rouzaud, F., Kadekaro, A. L., Abdel-Malek, Z. A. & Hearing, V. J. MC1R and the response of melanocytes to ultraviolet radiation. Mutat. Res. 571, 133–152 (2005)
Wenczl, E. et al. (Pheo)melanin photosensitizes UVA-induced DNA damage in cultured human melanocytes. J. Invest. Dermatol. 111, 678–682 (1998)
Hill, H. Z. & Hill, G. J. UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Res. 13 (suppl. 8). 140–144 (2000)
Curtin, J. A. et al. Distinct sets of genetic alterations in melanoma. N. Engl. J. Med. 353, 2135–2147 (2005)
Elwood, J. M. & Jopson, J. Melanoma and sun exposure: an overview of published studies. Int. J. Cancer 73, 198–203 (1997)
Robbins, L. S. et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 72, 827–834 (1993)
Halaban, R. et al. Tyrosinases of murine melanocytes with mutations at the albino locus. Proc. Natl Acad. Sci. USA 85, 7241–7245 (1988)
Vanover, J. C. et al. Stem cell factor rescues tyrosinase expression and pigmentation in discreet anatomic locations in albino mice. Pigment Cell Melanoma Res. 22, 827–838 (2009)
Kunisada, T. et al. Murine cutaneous mastocytosis and epidermal melanocytosis induced by keratinocyte expression of transgenic stem cell factor. J. Exp. Med. 187, 1565–1573 (1998)
Dankort, D. et al. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nature Genet. 41, 544–552 (2009)
Patton, E. E. et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 15, 249–254 (2005)
Goel, V. K. et al. Melanocytic nevus-like hyperplasia and melanoma in transgenic BRAFV600E mice. Oncogene 28, 2289–2298 (2009)
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005)
Dhomen, N. et al. Oncogenic Braf induces melanocyte senescence and melanoma in mice. Cancer Cell 15, 294–303 (2009)
Rae, J. et al. V600EBraf::Tyr-CreERT2::K14-Kitl mice do not develop superficial spreading-like melanoma: keratinocyte kit ligand is insufficient to “translocate” V600EBraf-driven melanoma to the epidermis. J. Invest. Dermatol. 132, 488–491 (2012)
D'Orazio, J. A. et al. Topical drug rescue strategy and skin protection based on the role of Mc1r in UV-induced tanning. Nature 443, 340–344 (2006)
Boni, A. et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 70, 5213–5219 (2010)
Kadekaro, A. L. et al. Melanocortin 1 receptor genotype: an important determinant of the damage response of melanocytes to ultraviolet radiation. FASEB J. 24, 3850–3860 (2010)
Noonan, F. P. et al. Melanoma induction by ultraviolet A but not ultraviolet B radiation requires melanin pigment. Nature Commun. 3, 884 (2012)
Nofsinger, J. B., Liu, Y. & Simon, J. D. Aggregation of eumelanin mitigates photogeneration of reactive oxygen species. Free Radic. Biol. Med. 32, 720–730 (2002)
Kovacs, D. et al. The eumelanin intermediate 5,6-dihydroxyindole-2-carboxylic acid is a messenger in the cross-talk among epidermal cells. J. Invest. Dermatol. 132, 1196–1205 (2012)
Wang, J. et al. Quantification of oxidative DNA lesions in tissues of Long-Evans Cinnamon rats by capillary high-performance liquid chromatography−tandem mass spectrometry coupled with stable isotope-dilution method. Anal. Chem. 83, 2201–2209 (2011)
Wang, Y. Bulky DNA lesions induced by reactive oxygen species. Chem. Res. Toxicol. 21, 276–281 (2008)
Jaruga, P. & Dizdaroglu, M. 8,5′-Cyclopurine-2′-deoxynucleosides in DNA: mechanisms of formation, measurement, repair and biological effects. DNA Repair (Amst.) 7, 1413–1425 (2008)
Yuan, B., Wang, J., Cao, H., Sun, R. & Wang, Y. High-throughput analysis of the mutagenic and cytotoxic properties of DNA lesions by next-generation sequencing. Nucleic Acids Res. 39, 5945–5954 (2011)
Neugut, A. I., Kizelnik-Freilich, S. & Ackerman, C. Black-white differences in risk for cutaneous, ocular, and visceral melanomas. Am. J. Public Health 84, 1828–1829 (1994)
Green, A. C., Williams, G. M., Logan, V. & Strutton, G. M. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J. Clin. Oncol. 29, 257–263 (2011)
Huncharek, M. & Kupelnick, B. Use of topical sunscreens and the risk of malignant melanoma: a meta-analysis of 9067 patients from 11 case-control studies. Am. J. Public Health 92, 1173–1177 (2002)
Acknowledgements
We thank T. Kunisada for generously sharing K14-SCF mice and C. L. Evans for help with mouse skin irradiation. We also thank A. P. Codgill for help with primary tumour cell culture and A. Piris for pathology consultation as well as M. Haigis and Z. Abdel-Malik for discussions. This work was supported by the following grants from the National Institutes of Health 5R01 AR043369-16 (D.E.F.), R01-CA101864 (Y.W.) and F30 ES020663-01 (D.M.), as well as support from the Dr Miriam and Sheldon G. Adelson Medical Research Foundation, the US-Israel Binational Science Foundation, and the Melanoma Research Alliance (D.E.F.).
Author information
Authors and Affiliations
Contributions
D.M. and D.E.F. conceived and planned the project. M.M., M.W.B. and K.M.H. provided the mice carrying the PTEN, BRAFV600E and Tyr-Cre(ER)T2 alleles. D.M. performed the mouse work with help from A.M., J.L., S.P.D. and K.C.R. Histology was performed by D.M., X.L., J.C.V. and J.A.D. with support from D.A.H. Pathological analysis was provided by M.P.H., J.K.L. and M.C.M. In vitro studies were performed by D.M. with help from A.M. and J.L. J.A.W. generated the primary mouse cell line. J.W., C.R.G. and Y.W. collected DNA from mouse skin and performed LC–MS/MS/MS. The manuscript was written by D.M. and D.E.F. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1-5. (PDF 5956 kb)
Rights and permissions
About this article
Cite this article
Mitra, D., Luo, X., Morgan, A. et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature 491, 449–453 (2012). https://doi.org/10.1038/nature11624
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11624
This article is cited by
-
Low incidence of BRAF and NRAS mutations in a population with a high incidence of melanoma
Virchows Archiv (2024)
-
Alpha-melanocyte stimulating hormone (α-MSH): biology, clinical relevance and implication in melanoma
Journal of Translational Medicine (2023)
-
The journey from melanocytes to melanoma
Nature Reviews Cancer (2023)
-
Establishment of SLC7A11‐knockout mouse and its preliminary investigation in melanoma
In Vitro Cellular & Developmental Biology - Animal (2023)
-
Dermatoskopie nichtneoplastischer Erkrankungen auf dunkler Haut
Die Dermatologie (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.