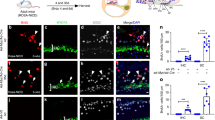
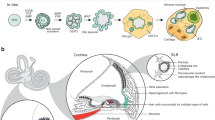
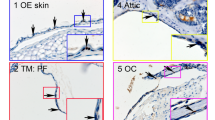
Abstract
Deafness is a condition with a high prevalence worldwide, produced primarily by the loss of the sensory hair cells and their associated spiral ganglion neurons (SGNs). Of all the forms of deafness, auditory neuropathy is of particular concern. This condition, defined primarily by damage to the SGNs with relative preservation of the hair cells1, is responsible for a substantial proportion of patients with hearing impairment2. Although the loss of hair cells can be circumvented partially by a cochlear implant, no routine treatment is available for sensory neuron loss, as poor innervation limits the prospective performance of an implant3. Using stem cells to recover the damaged sensory circuitry is a potential therapeutic strategy. Here we present a protocol to induce differentiation from human embryonic stem cells (hESCs) using signals involved in the initial specification of the otic placode. We obtained two types of otic progenitors able to differentiate in vitro into hair-cell-like cells and auditory neurons that display expected electrophysiological properties. Moreover, when transplanted into an auditory neuropathy model, otic neuroprogenitors engraft, differentiate and significantly improve auditory-evoked response thresholds. These results should stimulate further research into the development of a cell-based therapy for deafness.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vlastarakos, P. V., Nikolopoulos, T. P., Tavoulari, E., Papacharalambous, G. & Korres, S. Auditory neuropathy: endocochlear lesion or temporal processing impairment? Implications for diagnosis and management. Int. J. Pediatr. Otorhinolaryngol. 72, 1135–1150 (2008)
Uus, K. & Bamford, J. Effectiveness of population-based newborn hearing screening in England: ages of interventions and profile of cases. Pediatrics 117, e887–e893 (2006)
Bradley, J., Beale, T., Graham, J. & Bell, M. Variable long-term outcomes from cochlear implantation in children with hypoplastic auditory nerves. Cochlear Implants Int. 9, 34–60 (2008)
Li, H., Liu, H. & Heller, S. Pluripotent stem cells from the adult mouse inner ear. Nature Med. 9, 1293–1299 (2003)
Li, H., Roblin, G., Liu, H. & Heller, S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc. Natl Acad. Sci. USA 100, 13495–13500 (2003)
Oshima, K. et al. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell 141, 704–716 (2010)
Jeon, S. J., Oshima, K., Heller, S. & Edge, A. S. Bone marrow mesenchymal stem cells are progenitors in vitro for inner ear hair cells. Mol. Cell. Neurosci. 34, 59–68 (2007)
Kondo, T., Johnson, S. A., Yoder, M. C., Romand, R. & Hashino, E. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc. Natl Acad. Sci. USA 102, 4789–4794 (2005)
Coleman, B., Fallon, J. B., Pettingill, L. N., de Silva, M. G. & Shepherd, R. K. Auditory hair cell explant co-cultures promote the differentiation of stem cells into bipolar neurons. Exp. Cell Res. 313, 232–243 (2007)
Reyes, J. H. et al. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J. Neurosci. 28, 12622–12631 (2008)
Corrales, C. E. et al. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: growth of processes into the organ of Corti. J. Neurobiol. 66, 1489–1500 (2006)
Hildebrand, M. S. et al. Survival of partially differentiated mouse embryonic stem cells in the scala media of the guinea pig cochlea. J. Assoc. Res. Otolaryngol. 6, 341–354 (2005)
Sekiya, T. et al. Transplantation of conditionally immortal auditory neuroblasts to the auditory nerve. Eur. J. Neurosci. 25, 2307–2318 (2007)
Lang, H. et al. Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury. J. Assoc. Res. Otolaryngol. 9, 225–240 (2008)
Hu, Z., Ulfendahl, M. & Olivius, N. P. Central migration of neuronal tissue and embryonic stem cells following transplantation along the adult auditory nerve. Brain Res. 1026, 68–73 (2004)
Rask-Andersen, H. et al. Regeneration of human auditory nerve. In vitro/in video demonstration of neural progenitor cells in adult human and guinea pig spiral ganglion. Hear. Res. 203, 180–191 (2005)
Shi, F., Corrales, C. E., Liberman, M. C. & Edge, A. S. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur. J. Neurosci. 26, 3016–3023 (2007)
Lee, G. et al. Isolation and directed differentiation of neural crest stem cells derived from human embryonic stem cells. Nature Biotechnol. 25, 1468–1475 (2007)
Chen, W. et al. Human fetal auditory stem cells can be expanded in vitro and differentiate into functional auditory neurons and hair cell-like cells. Stem Cells 27, 1196–1204 (2009)
Martin, K. & Groves, A. K. Competence of cranial ectoderm to respond to Fgf signaling suggests a two-step model of otic placode induction. Development 133, 877–887 (2006)
Freter, S., Muta, Y., Mak, S. S., Rinkwitz, S. & Ladher, R. K. Progressive restriction of otic fate: the role of FGF and Wnt in resolving inner ear potential. Development 135, 3415–3424 (2008)
Alvarez, Y. et al. Requirements for FGF3 and FGF10 during inner ear formation. Development 130, 6329–6338 (2003)
Wright, T. J. & Mansour, S. L. Fgf3 and Fgf10 are required for mouse otic placode induction. Development 130, 3379–3390 (2003)
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005)
Schmiedt, R. A., Okamura, H. O., Lang, H. & Schulte, B. A. Ouabain application to the round window of the gerbil cochlea: a model of auditory neuropathy and apoptosis. J. Assoc. Res. Otolaryngol. 3, 223–233 (2002)
Lang, H., Schulte, B. A. & Schmiedt, R. A. Ouabain induces apoptotic cell death in type I spiral ganglion neurons, but not type II neurons. J. Assoc. Res. Otolaryngol. 6, 63–74 (2005)
McLean, W. J., Smith, K. A., Glowatzki, E. & Pyott, S. J. Distribution of the Na,K-ATPase alpha subunit in the rat spiral ganglion and organ of corti. J. Assoc. Res. Otolaryngol. 10, 37–49 (2009)
Burkard, R., Boettcher, F., Voigt, H. & Mills, J. Comments on “Stimulus dependencies of the gerbil brain-stem auditory-evoked response (BAER). I: Effects of click level, rate and polarity” [J. Acoust. Soc. Am. 85, 2514–2525 (1989)]. J. Acoust. Soc. Am. 94, 2441–2442 (1993)
Boettcher, F. A., Mills, J. H. & Norton, B. L. Age-related changes in auditory evoked potentials of gerbils. I. Response amplitudes. Hear. Res. 71, 137–145 (1993)
Acknowledgements
This work was supported primarily by grants from Action on Hearing Loss (RNID) to M.N.R. Other support included Deafness Research UK (M.N.R and W.M.), the Wellcome Trust (088719, W.M.), Medical Research Council (P.W.A, H.D.M. and M.N.R) and ESTOOLS (P.W.A). S.L.J. was supported by a Wellcome Trust VIP award and the RNID. W.M. and S.L.J. are Royal Society university research fellows. Confocal images were taken at the Light Microscopy Facility of the Department of Biomedical Sciences, University of Sheffield. We are grateful for the advice of M. Mulheran and I. Russell on the tests of auditory function, provided at the earlier stages of this project, and to the assistance of P. Gokhale on the use of the InCell Analyzer. M.N.R. acknowledges the support and encouragement of his late parents, Noemí Luján-Ceballos and Juan Carlos Rivolta.
Author information
Authors and Affiliations
Contributions
W.C., N.J., L.A., S.J.E and J.K.T. collected and/or assembled, analysed and interpreted data. S.L.J., S.K. and W.M. collected and/or assembled, analysed and intepreted electrophysiology data. M.M. carried out biocomputational analysis of gene-array data. P.W.A. and H.D.M. provided study material and administrative support. M.N.R. was responsible for conceiving and designing the study, for obtaining financial support, collecting and/or assembling, analysing and interpreting data, for manuscript writing, and for final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1 and 2 and Supplementary Figures 1-15. (PDF 1936 kb)
Supplementary Data
This zipped file contains Supplementary Tables 3-10. Supplementary Table 3 contains the genes differentially upregulated in the FGF condition when compared to undifferentiated hESCs, using a threshold of 1.5fold-change. Supplementary Table 4 contains the genes differentially upregulated in the FGF condition when compared to cells in DFNB, using a threshold of 1.5fold-change. Supplementary Table 5 contains the genes differentially downregulated in the FGF condition when compared to undifferentiated hESCs, using a threshold of 1.5fold-change. Supplementary Table 6 contains the genes differentially downregulated in the FGF condition when compared to cells in DFNB, using a threshold of 1.5fold-change. Supplementary Table 7 the top Gene Ontology BP5 terms enriched in the genes upregulated by a 1.5 fold-change in FGF when compared to undifferentiated hESCs. Supplementary Table 8 the top Gene Ontology BP5 terms enriched in the genes upregulated by a 1.5 fold-change in FGF when compared to cells in DFNB. Supplementary Table 9 contains the top Gene Ontology BP5 terms enriched in the genes downregulated by a 1.5 fold-change in FGF when compared to undifferentiated hESCs. Supplementary Table 10 contains the top Gene Ontology BP5 terms enriched in the genes downregulated by a 1.5 fold-change in FGF when compared to cells in DFNB. (ZIP 913 kb)
Rights and permissions
About this article
Cite this article
Chen, W., Jongkamonwiwat, N., Abbas, L. et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature 490, 278–282 (2012). https://doi.org/10.1038/nature11415
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11415
This article is cited by
-
Stem Cell-Based Hair Cell Regeneration and Therapy in the Inner Ear
Neuroscience Bulletin (2024)
-
Stem cell therapy in sensorineural hearing loss: a systematic review
The Egyptian Journal of Otolaryngology (2023)
-
Enhanced survival of hypoimmunogenic otic progenitors following intracochlear xenotransplantation: repercussions for stem cell therapy in hearing loss models
Stem Cell Research & Therapy (2023)
-
Impaired AIF-CHCHD4 interaction and mitochondrial calcium overload contribute to auditory neuropathy spectrum disorder in patient-iPSC-derived neurons with AIFM1 variant
Cell Death & Disease (2023)
-
Cochlear Health and Cochlear-implant Function
Journal of the Association for Research in Otolaryngology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.