Abstract
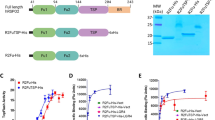
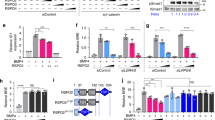
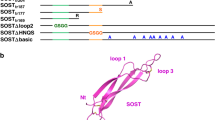
R-spondin proteins strongly potentiate Wnt signalling and function as stem-cell growth factors. Despite the biological and therapeutic significance, the molecular mechanism of R-spondin action remains unclear. Here we show that the cell-surface transmembrane E3 ubiquitin ligase zinc and ring finger 3 (ZNRF3) and its homologue ring finger 43 (RNF43) are negative feedback regulators of Wnt signalling. ZNRF3 is associated with the Wnt receptor complex, and inhibits Wnt signalling by promoting the turnover of frizzled and LRP6. Inhibition of ZNRF3 enhances Wnt/β-catenin signalling and disrupts Wnt/planar cell polarity signalling in vivo. Notably, R-spondin mimics ZNRF3 inhibition by increasing the membrane level of Wnt receptors. Mechanistically, R-spondin interacts with the extracellular domain of ZNRF3 and induces the association between ZNRF3 and LGR4, which results in membrane clearance of ZNRF3. These data suggest that R-spondin enhances Wnt signalling by inhibiting ZNRF3. Our study provides new mechanistic insights into the regulation of Wnt receptor turnover, and reveals ZNRF3 as a tractable target for therapeutic exploration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 (2006)
MacDonald, B. T., Tamai, K. & He, X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26 (2009)
Simons, M. & Mlodzik, M. Planar cell polarity signaling: from fly development to human disease. Annu. Rev. Genet. 42, 517–540 (2008)
Kazanskaya, O. et al. R-Spondin2 is a secreted activator of Wnt/β-catenin signaling and is required for Xenopus myogenesis. Dev. Cell 7, 525–534 (2004)
Kim, K. A. et al. Mitogenic influence of human R-spondin1 on the intestinal epithelium. Science 309, 1256–1259 (2005)
Kim, K. A. et al. R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell 19, 2588–2596 (2008)
Ohkawara, B., Glinka, A. & Niehrs, C. Rspo3 binds syndecan 4 and induces Wnt/PCP signaling via clathrin-mediated endocytosis to promote morphogenesis. Dev. Cell 20, 303–314 (2011)
Aoki, M. et al. R-spondin3 is required for mouse placental development. Dev. Biol. 301, 218–226 (2007)
Blaydon, D. C. et al. The gene encoding R-spondin 4 (RSPO4), a secreted protein implicated in Wnt signaling, is mutated in inherited anonychia. Nature Genet. 38, 1245–1247 (2006)
Kazanskaya, O. et al. The Wnt signaling regulator R-spondin 3 promotes angioblast and vascular development. Development 135, 3655–3664 (2008)
Parma, P. et al. R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nature Genet. 38, 1304–1309 (2006)
Sato, T. et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011)
Ootani, A. et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nature Med. 15, 701–706 (2009)
Zhao, J. et al. R-Spondin1 protects mice from chemotherapy or radiation-induced oral mucositis through the canonical Wnt/β-catenin pathway. Proc. Natl Acad. Sci. USA 106, 2331–2336 (2009)
Nam, J. S., Turcotte, T. J., Smith, P. F., Choi, S. & Yoon, J. K. Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate β-catenin-dependent gene expression. J. Biol. Chem. 281, 13247–13257 (2006)
Wei, Q. et al. R-spondin1 is a high affinity ligand for LRP6 and induces LRP6 phosphorylation and β-catenin signaling. J. Biol. Chem. 282, 15903–15911 (2007)
Binnerts, M. E. et al. R-Spondin1 regulates Wnt signaling by inhibiting internalization of LRP6. Proc. Natl Acad. Sci. USA 104, 14700–14705 (2007)
Carmon, K. S., Gong, X., Lin, Q., Thomas, A. & Liu, Q. R-spondins function as ligands of the orphan receptors LGR4 and LGR5 to regulate Wnt/β-catenin signaling. Proc. Natl Acad. Sci. USA 108, 11452–11457 (2011)
de Lau, W. et al. Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 (2011)
Glinka, A. et al. LGR4 and LGR5 are R-spondin receptors mediating Wnt/b-catenin and Wnt/PCP signalling. EMBO Rep. 12, 1055–1061 (2011)
Chen, B. et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nature Chem. Biol. 5, 100–107 (2009)
Gonzalez-Sancho, J. M., Brennan, K. R., Castelo-Soccio, L. A. & Brown, A. M. Wnt proteins induce dishevelled phosphorylation via an LRP5/6- independent mechanism, irrespective of their ability to stabilize beta-catenin. Mol. Cell. Biol. 24, 4757–4768 (2004)
Gurney, A. L. Frizzled-binding agents and uses thereof. US patent 201037041. (2011)
Mukai, A. et al. Balanced ubiquitylation and deubiquitylation of Frizzled regulate cellular responsiveness to Wg/Wnt. EMBO J. 29, 2114–2125 (2010)
Kim, C. H. et al. Repressor activity of Headless/Tcf3 is essential for vertebrate head formation. Nature 407, 913–916 (2000)
Nasevicius, A. et al. Evidence for a frizzled-mediated wnt pathway required for zebrafish dorsal mesoderm formation. Development 125, 4283–4292 (1998)
Smith, A. N., Miller, L. A., Song, N., Taketo, M. M. & Lang, R. A. The duality of β-catenin function: a requirement in lens morphogenesis and signaling suppression of lens fate in periocular ectoderm. Dev. Biol. 285, 477–489 (2005)
Kreslova, J. et al. Abnormal lens morphogenesis and ectopic lens formation in the absence of β-catenin function. Genesis 45, 157–168 (2007)
Machon, O. et al. Lens morphogenesis is dependent on Pax6-mediated inhibition of the canonical Wnt/β-catenin signaling in the lens surface ectoderm. Genesis 48, 86–95 (2010)
Wang, J. et al. Dishevelled genes mediate a conserved mammalian PCP pathway to regulate convergent extension during neurulation. Development 133, 1767–1778 (2006)
Wu, J. et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc. Natl Acad. Sci. USA 108, 21188–21193 (2011)
Huang, S. M. et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 461, 614–620 (2009)
Zhang, Y. et al. RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin degradation and Wnt signalling. Nature Cell Biol. 13, 623–629 (2011)
Nusslein-Volhard, C. & Dahm, R. Zebrafish. A Practical Approach. (Oxford Univ. Press, 2002)
Westerfield, M. The Zebrafish Book: a Guide for the Laboratory Use of Zebrafish (Brachydanio rerio). (Univ. Oregon Press, 1995)
Goentoro, L. & Kirschner, M. W. Evidence that fold-change, and not absolute level, of β-catenin dictates Wnt signaling. Mol. Cell 36, 872–884 (2009)
Gerdes, J. M. et al. Disruption of the basal body compromises proteasomal function and perturbs intracellular Wnt response. Nature Genet. 39, 1350–1360 (2007)
Acknowledgements
We thank G. Yu, T. Lewis, Q. Song, J. Garver, J. Wang, B. Lu, B. Guo, Q. Fang, X. Shi, J. Sprunger and R.Freeman for technical assistance, R.-F. Kwong and T. Fleming for generating ZNRF3 antibodies, K. Lee and J. Halupowski for mouse maintenance, and J. Tchorz, A. Jaffe, N. Kubica, M. Hild, J. Solomon, Y. Yang, J. Tchorz, E. Wiellette, G. Michaud, D. Cutis and K. Seuwen for comments and advice. We also thank S. Goto for providing FZD4 and FZD4 K0 plasmids.
Author information
Authors and Affiliations
Contributions
H.-X.H. initiated the project, characterized the function of ZNRF3 in cultured cells and mice, and identified ZNRF3 antagonistic antibodies. H.-X.H. and Y.X. discovered the R-spondin and ZNRF3 link. Y.X. led mechanistic studies on R-spondin, LGR4 and ZNRF3. H.-X.H., Y.X., Y.Z., H.L., C.M., D.L., H.R., X.M., Q.M., T.B., P.M.F., M.W.K., J.A.P., F.C.S. and F.C. conceived and designed the study. H.-X.H., Y.X., Y.Z., O.C., E.O., M.A., H.L., C.M., D.L., H.R., X.M., Q.M., R.Z., F.C.S. and F.C. designed and implemented experiments. H.-X.H., Y.X., Y.Z. and F.C. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This fie contains Supplementary Figures 1-15. (PDF 2351 kb)
Rights and permissions
About this article
Cite this article
Hao, HX., Xie, Y., Zhang, Y. et al. ZNRF3 promotes Wnt receptor turnover in an R-spondin-sensitive manner. Nature 485, 195–200 (2012). https://doi.org/10.1038/nature11019
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11019
This article is cited by
-
R-spondin-1 induces Axin degradation via the LRP6-CK1ε axis
Cell Communication and Signaling (2024)
-
R-Spondin 2 governs Xenopus left-right body axis formation by establishing an FGF signaling gradient
Nature Communications (2024)
-
Role of the Ror family receptors in Wnt5a signaling
In Vitro Cellular & Developmental Biology - Animal (2024)
-
New insights in ubiquitin-dependent Wnt receptor regulation in tumorigenesis
In Vitro Cellular & Developmental Biology - Animal (2024)
-
LGR4 and LGR5 form distinct homodimers that only LGR4 complexes with RNF43/ZNRF3 to provide high affinity binding of R-spondin ligands
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.