Abstract
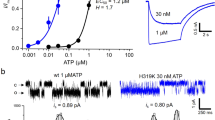
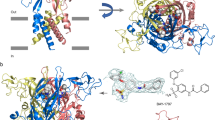
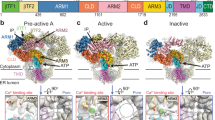
P2X receptors are trimeric ATP-activated ion channels permeable to Na+, K+ and Ca2+. The seven P2X receptor subtypes are implicated in physiological processes that include modulation of synaptic transmission, contraction of smooth muscle, secretion of chemical transmitters and regulation of immune responses. Despite the importance of P2X receptors in cellular physiology, the three-dimensional composition of the ATP-binding site, the structural mechanism of ATP-dependent ion channel gating and the architecture of the open ion channel pore are unknown. Here we report the crystal structure of the zebrafish P2X4 receptor in complex with ATP and a new structure of the apo receptor. The agonist-bound structure reveals a previously unseen ATP-binding motif and an open ion channel pore. ATP binding induces cleft closure of the nucleotide-binding pocket, flexing of the lower body β-sheet and a radial expansion of the extracellular vestibule. The structural widening of the extracellular vestibule is directly coupled to the opening of the ion channel pore by way of an iris-like expansion of the transmembrane helices. The structural delineation of the ATP-binding site and the ion channel pore, together with the conformational changes associated with ion channel gating, will stimulate development of new pharmacological agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
09 May 2012
A typographical error was corrected in the abstract; an addition was made to Acknowledgements.
References
Burnstock, G. Purinergic nerves. Pharmacol. Rev. 24, 509–581 (1972)
Valera, S. et al. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature 371, 516–519 (1994)
Webb, T. E. et al. Cloning and functional expression of a brain G-protein-coupled ATP receptor. FEBS Lett. 324, 219–225 (1993)
Surprenant, A. & North, R. A. Signaling at purinergic P2X receptors. Annu. Rev. Physiol. 71, 333–359 (2009)
Burnstock, G. & Kennedy, C. P2X receptors in health and disease. Adv. Pharmacol. 61, 333–372 (2011)
Coddou, C., Yan, Z., Obsil, T., Huidobro-Toro, J. P. & Stojikovic, S. S. Activation and regulation of purinergic P2X receptor channels. Pharmacol. Rev. 63, 641–683 (2011)
North, R. A. Molecular physiology of P2X receptors. Physiol. Rev. 82, 1013–1067 (2002)
Nicke, A. et al. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 17, 3016–3028 (1998)
Aschrafi, A., Sadtler, S., Niculescu, C., Rettinger, J. & Schmalzing, G. Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J. Mol. Biol. 342, 333–343 (2004)
Brake, A. J., Wagenbach, M. J. & Julius, D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature 371, 519–523 (1994)
Browne, L. E., Jiang, L. H. & North, R. A. New structure enlivens interest in P2X receptors. Trends Pharmacol. Sci. 31, 229–237 (2010)
Khakh, B. S., Bao, X. R., Labarca, C. & Lester, H. A. Neuronal P2X transmitter-gated cation channels change their ion selectivity in seconds. Nature Neurosci. 2, 322–330 (1999)
Virginio, C., MacKenzie, A., Rassendren, F. A., North, R. A. & Surprenant, A. Pore dilation of neuronal P2X receptor channels. Nature Neurosci. 2, 315–321 (1999)
Jarvis, M. F. & Khakh, B. S. ATP-gated P2X cation-channels. Neuropharmacology 56, 208–215 (2009)
Kucenas, S., Li, Z., Cox, J. A., Egan, T. M. & Voigt, M. M. Molecular characterization of the zebrafish P2X receptor subunit gene family. Neuroscience 121, 935–945 (2003)
Kawate, T., Michel, J. C., Birdsong, W. T. & Gouaux, E. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature 460, 592–598 (2009)
Jiang, L. H., Rassendren, F., Surprenant, A. & North, R. A. Identification of amino acid reisdues contributing to the ATP-binding site of a purinergic P2X receptor. J. Biol. Chem. 275, 34190–34196 (2000)
Ennion, S., Hagan, S. & Evans, R. J. The role of positively charged amino acids in ATP recognition by human P2X1 receptors. J. Biol. Chem. 275, 29361–29367 (2000)
Marquez-Klaka, B., Rettinger, J., Bhargava, Y., Eisele, T. & Nicke, A. Identification of an intersubunit cross-link between substituted cysteine residues located in the putative ATP binding site of the P2X1 receptor. J. Neurosci. 27, 1456–1466 (2007)
Roberts, J. A. & Evans, R. J. Cysteine substitution mutants give structural insight and identify ATP binding and activation sites at P2X receptors. J. Neurosci. 27, 4072–4082 (2007)
Roberts, J. A. et al. Cysteine substitution mutagenesis and the effects of methanethiosulfonate reagents at P2X2 and P2X4 receptors support a core common mode of ATP action at P2X receptors. J. Biol. Chem. 283, 20126–20136 (2008)
Bodnar, M. et al. Amino acid residues constituting the agonist binding site of the human P2X3 receptor. J. Biol. Chem. 286, 2739–2749 (2011)
Kawate, T. & Gouaux, E. Fluorescence-detection size-exclusion chromatography for precrystallization screening of integral membrane proteins. Structure 14, 673–681 (2006)
Wilkinson, W. J., Jiang, L. H., Surprenant, A. & North, R. A. Role of ectodomain lysines in the subunits of the heteromeric P2X2/3 receptor. Mol. Pharmacol. 70, 1159–1163 (2006)
Cavarelli, J. et al. The active site of yeast aspartyl-tRNA synthetase: structural and functional aspects of the aminoacylation reaction. EMBO J. 13, 327–337 (1994)
Gever, J. R., Cockayne, D. A., Dillon, M. P., Burnstock, G. & Ford, A. P. D. W. Pharmacology of P2X channels. Pflugers Arch. 452, 513–537 (2006)
Evans, R. J. et al. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors). Mol. Pharmacol. 48, 178–183 (1995)
Wildman, S. S., Brown, S. G., King, B. F. & Burnstock, G. Selectivity of diadenosine polyphosphates for rat P2X receptor subunits. Eur. J. Pharmacol. 367, 119–123 (1999)
Roberts, J. A., Valente, M., Allsopp, R. C., Watt, D. & Evans, R. J. Contribution of the region Glu181 to Val200 of the extracellular loop of the human P2X1 receptor to agonist binding and gating revealed using cysteine scanning mutagenesis. J. Neurochem. 109, 1042–1052 (2009)
Jiang, R. et al. Agonist trapped in ATP-binding sites of the P2X2 receptor. Proc. Natl Acad. Sci. USA 108, 9066–9071 (2011)
Bianchi, B. R. et al. Pharmacological characterization of recombinant human and rat P2X receptor subtypes. Eur. J. Pharmacol. 376, 127–138 (1999)
Virginio, C., Robertson, G., Surprenant, A. & North, R. A. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3, and heteromeric P2X2/3 receptors. Mol. Pharmacol. 53, 969–973 (1998)
Soto, F. et al. P2X4: an ATP-activated ionotropic receptor cloned from rat brain. Proc. Natl Acad. Sci. USA 93, 3684–3688 (1996)
Garcia-Guzman, M., Soto, F., Gomez-Hernandez, J. M., Lund, P. E. & Stühmer, W. Characterization of recombinant human P2X4 receptor reveals pharmacological differences to the rat homologue. Mol. Pharmacol. 51, 109–118 (1997)
Egan, T. M., Haines, W. R. & Voigt, M. M. A domain contributing to the ion channel of ATP-gated P2X2 receptors identified by the substituted cysteine accessibility method. J. Neurosci. 18, 2350–2359 (1998)
Rassendren, F., Buell, G., Newbolt, A., North, R. A. & Surprenant, A. Identification of amino acid residues contributing to the pore of a P2X receptor. EMBO J. 16, 3446–3454 (1997)
Li, M., Chang, T. H., Silberberg, S. D. & Swartz, K. J. Gating the pore of P2X receptor channels. Nature Neurosci. 11, 883–887 (2008)
Li, M., Kawate, T., Silberberg, S. D. & Swartz, K. J. Pore-opening mechanism in trimeric P2X receptor channels. Nature Commun. 1, 1–7 (2010)
Kracun, S., Chaptal, V., Abramson, J. & Khakh, B. S. Gated access to the pore of a P2X receptor: structural implications for closed-open transitions. J. Biol. Chem. 285, 10110–10121 (2010)
Evans, R. J. et al. Ionic permeability of, and divalent cation effects on, two ATP-gated cation channels (P2X receptors) expressed in mammalian cells. J. Physiol. (Lond.) 497, 413–422 (1996)
Liu, D. M. & Adams, D. J. Ionic selectivity of native ATP-activated (P2X) receptor channels in dissociated neurones from rat parasympathetic ganglia. J. Physiol. (Lond.) 534, 423–435 (2001)
Villarroel, A., Burnashev, N. & Sakmann, B. Dimensions of the narrow portion of a recombinant NMDA receptor channel. Biophys. J. 68, 866–875 (1995)
Migita, K., Haines, W. R., Voigt, M. M. & Egan, T. M. Polar residues of the second transmembrane domain influence cation permeability of the ATP-gated P2X2 receptor. J. Biol. Chem. 276, 30934–30941 (2001)
Browne, L. E. et al. P2X receptor channels show threefold symmetry in ionic charge selectivity and unitary conductance. Nature Neurosci. 14, 17–18 (2011)
Kawate, T., Robertson, J. L., Li, M., Silberberg, S. D. & Swartz, K. J. Ion access pathway to the transmembrane pore in P2X receptor channels. J. Gen. Physiol. 137, 579–590 (2011)
Samways, D. S., Khakh, B. S., Dutertre, S. & Egan, T. M. Preferential use of unobstructed lateral portals as the access route to the pore of human ATP-gated ion channels (P2X receptors). Proc. Natl Acad. Sci. USA 108, 13800–13805 (2011)
Fujiwara, Y., Keceli, B., Nakajo, K. & Kubo, Y. Voltage- and [ATP]-dependent gating of the P2X2 ATP receptor channel. J. Gen. Physiol. 133, 93–109 (2009)
Jelínková, I. et al. Identification of P2X4 receptor-specific residues contributing to the ivermectin effects on channel activation. Biochem. Biophys. Res. Commun. 349, 619–625 (2006)
Silberberg, S. D., Li, M. & Swartz, K. J. Ivermectin interaction with transmembrane helices reveals widespread rearrangements during opening of P2X receptor channels. Neuron 54, 263–274 (2007)
Khakh, B. S., Proctor, W. R., Dunwiddie, T. V., Labarca, C. & Lester, H. A. Allosteric control of gating and kinetics at P2X4 receptor channels. J. Neurosci. 19, 7289–7299 (1999)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
McCoy, A. J. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr. D 63, 32–41 (2007)
Collaborative Computational Project, 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D 58, 1948–1954 (2002)
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Cryst. 26, 283–291 (1993)
Davis, I. W. et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 35, 375–383 (2007)
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Samsom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360 (1996)
Hayward, S. & Lee, R. A. Improvements in the analysis of domain motions in proteins from conformational change: DynDom version 1.50. J. Mol. Graph. Model. 21, 181–183 (2002)
Acknowledgements
We thank C. Alexander and D. C. Dawson for providing Xenopus oocytes, K. L. Dürr and T. Friedrich for providing the pCNA3.1x vector, L. Vaskalis for assistance with figure illustrations, K. J. Swartz and M. P. Kavanaugh for advice related to the oocyte experiments, and Gouaux laboratory members for discussions. We are also grateful to the staff at the Advanced Photon Source beamline 24-ID-C for help with X-ray data collection. This work was supported by a Japan Society for the Promotion of Science Postdoctoral Fellowship for Research Abroad (M.H.), and by the American Asthma Foundation (E.G.) and the NIH (E.G.). E.G. is an investigator with the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
M.H. and E.G. contributed to all aspects of the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Table 1, Supplementary Figures 1-16 and an additional reference. (PDF 11145 kb)
Supplementary Movie 1
This movie shows the structural conformational changes in the TM region of zfP2X4 between the apo, closed (ΔzfP2X4-B2) and the ATP-bound, open (ΔzfP2X4-C) states, viewed from the intracellular side. The colouring of scheme is the same as Fig 1a. (MOV 6040 kb)
Supplementary Movie 2
This movie shows the structural conformational changes of zfP2X4 between the apo, closed (ΔzfP2X4-B) and the ATP-bound, open (ΔzfP2X4-C) states, viewed perpendicular to the membrane plane. The couloring of scheme of the head (A), dorsal fin (B), left flipper (A), body and TM domains (A, B) is the same as in Fig. 2a. (MOV 7128 kb)
Rights and permissions
About this article
Cite this article
Hattori, M., Gouaux, E. Molecular mechanism of ATP binding and ion channel activation in P2X receptors. Nature 485, 207–212 (2012). https://doi.org/10.1038/nature11010
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature11010
This article is cited by
-
P2X7 Receptor: an Emerging Target in Alzheimer’s Disease
Molecular Neurobiology (2024)
-
P2X7 receptor activation leads to NLRP3-independent IL-1β release by human macrophages
Cell Communication and Signaling (2023)
-
Fast functional mapping of ligand-gated ion channels
Communications Biology (2023)
-
Chronic cough relief by allosteric modulation of P2X3 without taste disturbance
Nature Communications (2023)
-
The P2 purinoceptors in prostate cancer
Purinergic Signalling (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.