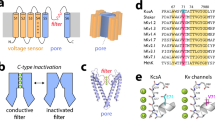
Abstract
A number of functionally important actions of proteins are mediated by short, intrinsically disordered peptide segments1, but the molecular interactions that allow disordered domains to mediate their effects remain a topic of active investigation2,3,4,5. Many K+ channel proteins, after initial channel opening, show a time-dependent reduction in current flux, termed ‘inactivation’, which involves movement of mobile cytosolic peptide segments (approximately 20–30 residues) into a position that physically occludes ion permeation6,7,8. Peptide segments that produce inactivation show little amino-acid identity6,9,10,11,12,13 and tolerate appreciable mutational substitutions13 without disrupting the inactivation process. Solution nuclear magnetic resonance of several isolated inactivation domains reveals substantial conformational heterogeneity with only minimal tendency to ordered structures14,15,16,17. Channel inactivation mechanisms may therefore help us to decipher how intrinsically disordered regions mediate functional effects. Whereas many aspects of inactivation of voltage-dependent K+ channels (Kv) can be described by a simple one-step occlusion mechanism6,7,18,19, inactivation of the voltage-dependent large-conductance Ca2+-gated K+ (BK) channel mediated by peptide segments of auxiliary β-subunits involves two distinguishable kinetic steps20,21. Here we show that two-step inactivation mediated by an intrinsically disordered BK β-subunit peptide involves a stereospecific binding interaction that precedes blockade. In contrast, blocking mediated by a Shaker Kv inactivation peptide is consistent with direct, simple occlusion by a hydrophobic segment without substantial steric requirement. The results indicate that two distinct types of molecular interaction between disordered peptide segments and their binding sites produce qualitatively similar functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
10.1017/S0033583511000060 Dyson, H. J. Expanding the proteome: disordered and alternatively folded proteins. Q. Rev. Biophys. 44, 467–518 (2011)
Sugase, K., Dyson, H. J. & Wright, P. E. Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature 447, 1021–1025 (2007)
Galea, C. A. et al. Role of intrinsic flexibility in signal transduction mediated by the cell cycle regulator, p27 Kip1. J. Mol. Biol. 376, 827–838 (2008)
Tompa, P. & Fuxreiter, M. Fuzzy complexes: polymorphism and structural disorder in protein–protein interactions. Trends Biochem. Sci. 33, 2–8 (2008)
De Sancho, D. & Best, R. B. Modulation of an IDP binding mechanism and rates by helix propensity and non-native interactions: association of HIF1α with CBP. Mol. Biosyst. 8, 256–267 (2012)
Hoshi, T., Zagotta, W. N. & Aldrich, R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250, 533–538 (1990)
Zagotta, W. N., Hoshi, T. & Aldrich, R. W. Restoration of inactivation in mutants of Shaker potassium channels by a peptide derived from ShB. Science 250, 568–571 (1990)
Zhou, M., Morais-Cabral, J. H., Mann, S. & MacKinnon, R. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature 411, 657–661 (2001)
Ruppersberg, J. P., Frank, R., Pongs, O. & Stocker, M. Cloned neuronal IK(A) channels reopen during recovery from inactivation. Nature 353, 657–660 (1991)
Tseng-Crank, J., Yao, J. A., Berman, M. F. & Tseng, G. N. Functional role of the NH2-terminal cytoplasmic domain of a mammalian A-type K channel. J. Gen. Physiol. 102, 1057–1083 (1993)
Rasmusson, R. L., Wang, S., Castellino, R. C., Morales, M. J. & Strauss, H. C. The beta subunit, Kv beta 1.2, acts as a rapid open channel blocker of NH2-terminal deleted Kv1.4 alpha-subunits. Adv. Exp. Med. Biol. 430, 29–37 (1997)
Kondoh, S., Ishii, K., Nakamura, Y. & Taira, N. A mammalian transient type K+ channel, rat Kv1.4, has two potential domains that could produce rapid inactivation. J. Biol. Chem. 272, 19333–19338 (1997)
Murrell-Lagnado, R. D. & Aldrich, R. W. Interactions of amino terminal domains of Shaker K channels with a pore blocking site studied with synthetic peptides. J. Gen. Physiol. 102, 949–975 (1993)
Schott, M. K., Antz, C., Frank, R., Ruppersberg, J. P. & Kalbitzer, H. R. Structure of the inactivating gate from the Shaker voltage gated K+ channel analyzed by NMR spectroscopy. Eur. Biophys. J. 27, 99–104 (1998)
Wissmann, R. et al. NMR structure and functional characteristics of the hydrophilic N terminus of the potassium channel β-subunit Kvβ1.1. J. Biol. Chem. 274, 35521–35525 (1999)
Wissmann, R. et al. Solution structure and function of the ‘tandem inactivation domain’ of the neuronal A-type potassium channel Kv1.4. J. Biol. Chem. 278, 16142–16150 (2003)
Bentrop, D., Beyermann, M., Wissmann, R. & Fakler, B. NMR structure of the ‘ball-and-chain’ domain of KCNMB2, the β2-subunit of large conductance Ca2+- and voltage-activated potassium channels. J. Biol. Chem. 276, 42116–42121 (2001)
Demo, S. D. & Yellen, G. The inactivation gate of the Shaker K+ channel behaves like an open-channel blocker. Neuron 7, 743–753 (1991)
Gonzalez, C., Lopez-Rodriguez, A., Srikumar, D., Rosenthal, J. J. & Holmgren, M. Editing of human KV1.1 channel mRNAs disrupts binding of the N-terminus tip at the intracellular cavity. Nature Commun. 2, 436, http://dx.doi.org/10.1038/ncomms1446 (2011)
Lingle, C. J., Zeng, X.-H., Ding, J.-P. & Xia, X.-M. Inactivation of BK channels mediated by the N-terminus of the β3b auxiliary subunit involves a two-step mechanism: possible separation of binding and blockade. J. Gen. Physiol. 117, 583–605 (2001)
Zeng, X.-H., Xia, X. M. & Lingle, C. J. BK channels with β3a subunits generate use-dependent slow afterhyperpolarizing currents by an inactivation-coupled mechanism. J. Neurosci. 27, 4707–4715 (2007)
Xia, X.-M., Ding, J. P. & Lingle, C. J. Molecular basis for the inactivation of Ca2+- and voltage-dependent BK channels in adrenal chromaffin cells and rat insulinoma tumor cells. J. Neurosci. 19, 5255–5264 (1999)
Wallner, M., Meera, P. & Toro, L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane β-subunit homolog. Proc. Natl Acad. Sci. USA 96, 4137–4142 (1999)
Xia, X.-M., Ding, J.-P., Zeng, X.-H., Duan, K.-L. & Lingle, C. J. Rectification and rapid activation at low Ca2+ of Ca2+-activated, voltage-dependent BK currents: consequences of rapid inactivation by a novel β subunit. J. Neurosci. 20, 4890–4903 (2000)
Prince-Carter, A. & Pfaffinger, P. J. Multiple intermediate states precede pore block during N-type inactivation of a voltage-gated potassium channel. J. Gen. Physiol. 134, 15–34 (2009)
Choi, K. L., Aldrich, R. W. & Yellen, G. Tetraethylammonium blockade distinguishes two inactivation mechanisms in voltage-activated K+ channels. Proc. Natl Acad. Sci. USA 88, 5092–5095 (1991)
Li, W. & Aldrich, R. W. Unique inner pore properties of BK channels revealed by quaternary ammonium block. J. Gen. Physiol. 124, 43–57 (2004)
Wilkens, C. M. & Aldrich, R. W. State-independent block of BK channels by an intracellular quaternary ammonium. J. Gen. Physiol. 128, 347–364 (2006)
Brelidze, T. I. & Magleby, K. L. Probing the geometry of the inner vestibule of BK channels with sugars. J. Gen. Physiol. 126, 105–121 (2005)
Zhou, Y., Xia, X. M. & Lingle, C. J. Cysteine scanning and modification reveal major differences between BK channels and Kv channels in the inner pore region. Proc. Natl Acad. Sci. USA 108, 12161–12166 (2011)
Tang, Q., Zeng, X.-H. & Lingle, C. J. Closed channel block of BK potassium channels by bbTBA requires partial activation. J. Gen. Physiol. 134, 409–436 (2009)
Zeng, X., Xia, X. M. & Lingle, C. J. Species-specific differences among KCNMB3 BK β3 auxiliary subunits: some β3 variants may be primate-specific subunits. J. Gen. Physiol. 132, 115–129 (2008)
Zeng, X.-H., Xia, X.-M. & Lingle, C. J. Redox-sensitive extracellular gates formed by auxiliary β subunits of calcium-activated potassium channels. Nature Struct. Biol. 10, 448–454 (2003)
Timpe, L. C. et al. Expression of functional potassium channels from Shaker cDNA in Xenopus oocytes. Nature 331, 143–145 (1988)
Hamill, O. P., Marty, A., Neher, E., Sakmann, B. & Sigworth, F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 391, 85–100 (1981)
Acknowledgements
This work was supported by GM-081748 to C.J.L. and the Searle Scholars Program to K.H.-W. We thank E. Morrison and P. Schlesinger for assistance with dynamic light scattering measurements, and C. Frieden and K. Garai for assistance with circular dichroism spectroscopy. We thank H. Jiang, A. Scott and J. Jones for care of oocytes, and J. H. Steinbach and R. Pappu for comments on the manuscript.
Author information
Authors and Affiliations
Contributions
V.G.-P. and X.-H.Z. designed experiments and collected and analysed data. K.H.-W. performed or supervised circular dichroism and NMR determinations. C.J.L. conceived the project, designed research, analysed data and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Text, Supplementary Figures 1-8 and Supplementary References. (PDF 3416 kb)
Rights and permissions
About this article
Cite this article
Gonzalez-Perez, V., Zeng, XH., Henzler-Wildman, K. et al. Stereospecific binding of a disordered peptide segment mediates BK channel inactivation. Nature 485, 133–136 (2012). https://doi.org/10.1038/nature10994
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10994
This article is cited by
-
A dynamic interaction process between KaiA and KaiC is critical to the cyanobacterial circadian oscillator
Scientific Reports (2016)
-
Quasi-specific access of the potassium channel inactivation gate
Nature Communications (2014)
-
Erratum: Stereospecific binding of a disordered peptide segment mediates BK channel inactivation
Nature (2012)
-
The BK channel: a vital link between cellular calcium and electrical signaling
Protein & Cell (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.