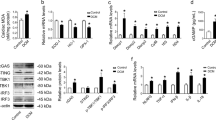
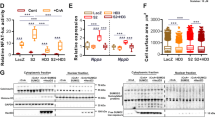
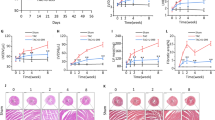
Abstract
Heart failure is a leading cause of morbidity and mortality in industrialized countries. Although infection with microorganisms is not involved in the development of heart failure in most cases, inflammation has been implicated in the pathogenesis of heart failure1. However, the mechanisms responsible for initiating and integrating inflammatory responses within the heart remain poorly defined. Mitochondria are evolutionary endosymbionts derived from bacteria and contain DNA similar to bacterial DNA2,3,4. Mitochondria damaged by external haemodynamic stress are degraded by the autophagy/lysosome system in cardiomyocytes5. Here we show that mitochondrial DNA that escapes from autophagy cell-autonomously leads to Toll-like receptor (TLR) 9-mediated inflammatory responses in cardiomyocytes and is capable of inducing myocarditis and dilated cardiomyopathy. Cardiac-specific deletion of lysosomal deoxyribonuclease (DNase) II showed no cardiac phenotypes under baseline conditions, but increased mortality and caused severe myocarditis and dilated cardiomyopathy 10 days after treatment with pressure overload. Early in the pathogenesis, DNase II-deficient hearts showed infiltration of inflammatory cells and increased messenger RNA expression of inflammatory cytokines, with accumulation of mitochondrial DNA deposits in autolysosomes in the myocardium. Administration of inhibitory oligodeoxynucleotides against TLR9, which is known to be activated by bacterial DNA6, or ablation of Tlr9 attenuated the development of cardiomyopathy in DNase II-deficient mice. Furthermore, Tlr9 ablation improved pressure overload-induced cardiac dysfunction and inflammation even in mice with wild-type Dnase2a alleles. These data provide new perspectives on the mechanism of genesis of chronic inflammation in failing hearts.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mann, D. L. Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ. Res. 91, 988–998 (2002)
Pollack, Y., Kasir, J., Shemer, R., Metzger, S. & Szyf, M. Methylation pattern of mouse mitochondrial DNA. Nucleic Acids Res. 12, 4811–4824 (1984)
Cardon, L., Burge, C., Clayton, D. A. & Karlin, S. Pervasive CpG suppression in animal mitochondrial genomes. Proc. Natl Acad. Sci. USA 91, 3799–3803 (1994)
Gray, M. W., Burger, G. & Lang, B. F. Mitochondrial evolution. Science 283, 1476–1481 (1999)
Nakai, A. et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nature Med. 13, 619–624 (2007)
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000)
Taanman, J.-W. The mitochondrial genome: structure, transcription, translation and replication. Biochim Biophys Acta Bioenerget. 1410, 103–123 (1999)
Collins, L., Hajizadeh, S., Holme, E., Jonsso, I. & Tarkowski, A. Endogenously oxidized mitochondrial DNA induces in vivo and in vitro inflammatory responses. J. Leukoc. Biol. 75, 995–1000 (2004)
Mizushima, N., Levine, B., Cuervo, A. M. & Klionsky, D. J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008)
Meerson, F., Zaletayeva, T., Lagutchev, S. & Pshennikova, M. Structure and mass of mitochondria in the process of compensatory hyperfunction and hypertrophy of the heart. Exp. Cell Res. 36, 568–578 (1964)
Bugger, H. et al. Proteomic remodelling of mitochondrial oxidative pathways in pressure overload-induced heart failure. Cardiovasc. Res. 85, 376–384 (2010)
Evans, C. J. & Aguilera, R. J. DNase II: genes, enzymes and function. Gene 322, 1–15 (2003)
Kawane, K. et al. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 443, 998–1002 (2006)
Ashley, N., Harris, D. & Poulton, J. Detection of mitochondrial DNA depletion in living human cells using PicoGreen staining. Exp. Cell Res. 303, 432–446 (2005)
Kabeya, Y. et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 19, 5720–5728 (2000)
Yamaguchi, O. et al. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J. Clin. Invest. 114, 937–943 (2004)
Lentz, S. I. et al. Mitochondrial DNA (mtDNA) biogenesis: visualization and duel incorporation of BrdU and EdU into newly synthesized mtDNA in vitro. J. Histochem. Cytochem. 58, 207–218 (2010)
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010)
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010)
Stunz, L. et al. Inhibitory oligonucleotides specifically block effects of stimulatory CpG oligonucleotides in B cells. Eur. J. Immunol. 32, 1212–1222 (2002)
Bianchi, M. E. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 (2007)
Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature Immunol. 12, 222–230 (2011)
Yamaguchi, O. et al. Targeted deletion of apoptosis signal-regulating kinase 1 attenuates left ventricular remodeling. Proc. Natl Acad. Sci. USA 100, 15883–15888 (2003)
Koizumi, T. Deoxyribonuclease II (DNase II) activity in mouse tissues and body fluids. Exp. Anim. 44, 169–171 (1995)
Lu, Z. et al. Participation of autophagy in the degeneration process of rat hepatocytes after transplantation following prolonged cold preservation. Arch. Histol. Cytol. 68, 71–80 (2005)
Mosgoller, W. et al. Distribution of DNA in human Sertoli cell nucleoli. J. Histochem. Cytochem. 41, 1487–1493 (1993)
Acknowledgements
We thank S. Nagata and K. Kawane, Kyoto University, for discussions and a gift of Dnase2aflox/flox mice, and Y. Uchiyama, Juntendo University, for anti-LC3 antibody. We also thank K. Takada for technical assistance. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology in Japan and research grants from Mitsubishi Pharma Research Foundation and the British Heart Foundation (CH/11/3/29051, RG/11/12/29052).
Author information
Authors and Affiliations
Contributions
S.A. and I.K. provided intellectual input; K.O. was responsible for the overall study design and writing the manuscript. The other authors performed experiments and analysed data. All authors contributed to the discussions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-10 and Supplementary Tables 1-2. (PDF 13510 kb)
Rights and permissions
About this article
Cite this article
Oka, T., Hikoso, S., Yamaguchi, O. et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485, 251–255 (2012). https://doi.org/10.1038/nature10992
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10992
This article is cited by
-
Control of NAD+ homeostasis by autophagic flux modulates mitochondrial and cardiac function
The EMBO Journal (2024)
-
Therapeutic Strategies Targeting Mitochondrial Dysfunction in Sepsis-induced Cardiomyopathy
Cardiovascular Drugs and Therapy (2024)
-
The Emerging Role of Toll-Like Receptor-Mediated Neuroinflammatory Signals in Psychiatric Disorders and Acquired Epilepsy
Molecular Neurobiology (2024)
-
Elevated cell-free mitochondria DNA level of patients with premature ovarian insufficiency
BMC Pregnancy and Childbirth (2023)
-
Emerging roles of mitochondria in animal regeneration
Cell Regeneration (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.