Abstract
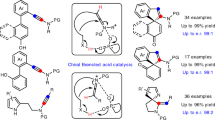
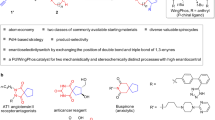
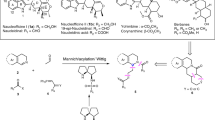
Acetals are molecular substructures that contain two oxygen–carbon single bonds at the same carbon atom, and are used in cells to construct carbohydrates and numerous other molecules. A distinctive subgroup are spiroacetals, acetals joining two rings, which occur in a broad range of biologically active compounds, including small insect pheromones and more complex macrocycles1,2. Despite numerous methods for the catalytic asymmetric formation of other commonly occurring stereocentres, there are few approaches that exclusively target the chiral acetal centre and none for spiroacetals3,4. Here we report the design and synthesis of confined Brønsted acids based on a C2-symmetric imidodiphosphoric acid motif, enabling a catalytic enantioselective spiroacetalization reaction. These rationally constructed Brønsted acids possess an extremely sterically demanding chiral microenvironment, with a single catalytically relevant and geometrically constrained bifunctional active site. Our catalyst design is expected to be of broad utility in catalytic asymmetric reactions involving small and structurally or functionally unbiased substrates.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
Data deposits
X-ray crystallographic data have been deposited in the Cambridge Crystallographic Data Centre database (http://www.ccdc.cam.ac.uk/) under accession code CCDC 864762.
References
Perron, F. & Albizati, K. F. Chemistry of spiroketals. Chem. Rev. 89, 1617–1661 (1989)
Aho, J. E., Pihko, P. M. & Rissa, T. K. Nonanomeric spiroketals in natural products: structures, sources, and synthetic strategies. Chem. Rev. 105, 4406–4440 (2005)
Čorić, I., Vellalath, S. & List, B. Catalytic asymmetric transacetalization. J. Am. Chem. Soc. 132, 8536–8537 (2010)
Nagano, H. & Katsuki, T. Stereocontrolled OH protection: asymmetric tetrahydrofuranylation. Chem. Lett. 31, 782–783 (2002)
Zinzalla, G., Milroy, L.-G. & Ley, S. V. Chemical variation of natural product-like scaffolds: design and synthesis of spiroketal derivatives. Org. Biomol. Chem. 4, 1977–2002 (2006)
Brasholz, M., Sörgel, S., Azap, C. & Reißig, H.-U. Rubromycins: structurally intriguing, biologically valuable, synthetically challenging antitumour antibiotics. Eur. J. Org. Chem. 3801–3814 (2007)
Haniotakis, G., Francke, W., Mori, K., Redlich, H. & Schurig, V. Sex-specific activity of (R)-(−)- and (S)- (+)-1,7-dioxaspiro[5.5]undecane, the major pheromone of Dacus oleae. J. Chem. Ecol. 12, 1559–1568 (1986)
Redlich, H. & Francke, W. Synthesis of enantiomerically pure 1,7-dioxaspiro[5.5]undecanes, pheromone components of the olive fly (Dacus oleae). Angew. Chem. Int. Ed. 23, 519–520 (1984)
Takahashi, S. et al. Reveromycin A biosynthesis uses RevG and RevJ for stereospecific spiroacetal formation. Nature Chem. Biol. 7, 461–468 (2011)
Takaoka, L. R., Buckmelter, A. J., LaCruz, T. E. & Rychnovsky, S. D. Rational synthesis of contra-thermodynamic spiroacetals by reductive cyclizations. J. Am. Chem. Soc. 127, 528–529 (2005)
Moilanen, S. B., Potuzak, J. S. & Tan, D. S. Stereocontrolled synthesis of spiroketals via Ti(Oi-Pr)4-mediated kinetic spirocyclization of glycal epoxides with retention of configuration. J. Am. Chem. Soc. 128, 1792–1793 (2006)
Audrain, H., Thorhauge, J., Hazell, R. G. & Jørgensen, K. A. A novel catalytic and highly enantioselective approach for the synthesis of optically active carbohydrate derivatives. J. Org. Chem. 65, 4487–4497 (2000)
Akiyama, T., Itoh, J., Yokota, K. & Fuchibe, K. Enantioselective Mannich-type reaction catalyzed by a chiral Brønsted acid. Angew. Chem. Int. Edn 43, 1566–1568 (2004)
Uraguchi, D. & Terada, M. Chiral Brønsted acid-catalyzed direct Mannich reactions via electrophilic activation. J. Am. Chem. Soc. 126, 5356–5357 (2004)
Akiyama, T. Stronger Brønsted acids. Chem. Rev. 107, 5744–5758 (2007)
Nakashima, D. & Yamamoto, H. Design of chiral N-triflyl phosphoramide as a strong chiral Brønsted acid and its application to asymmetric Diels-Alder reaction. J. Am. Chem. Soc. 128, 9626–9627 (2006)
Doyle, A. G. & Jacobsen, E. N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 107, 5713–5743 (2007)
Huang, Y., Unni, K. A., Thadani, A. N. & Rawal, V. H. Single enantiomers from a chiral-alcohol catalyst. Nature 424, 146 (2003)
Zhang, Q.-W. et al. Brønsted acid catalyzed enantioselective semipinacol rearrangement for the synthesis of chiral spiroethers. Angew. Chem. Int. Edn 48, 8572–8574 (2009)
Shenoy, S. R., Crisóstomo, F. R. P., Iwasawa, T. & Rebek, J., Jr Organocatalysis in a synthetic receptor with an inwardly directed carboxylic acid. J. Am. Chem. Soc. 130, 5658–5659 (2008)
Hastings, C. J., Pluth, M. D., Bergman, R. G. & Raymond, K. N. Enzymelike catalysis of the Nazarov cyclization by supramolecular encapsulation. J. Am. Chem. Soc. 132, 6938–6940 (2010)
Vellalath, S., Čorić, I. & List, B. N-Phosphinyl phosphoramide—a chiral Brønsted acid motif for the direct asymmetric N,O-acetalization of aldehydes. Angew. Chem. Int. Edn 49, 9749–9752 (2010)
Xu, F. et al. SPINOL-Derived phosphoric acids: synthesis and application in enantioselective Friedel-Crafts reaction of indoles with imines. J. Org. Chem. 75, 8677–8680 (2010)
Čorić, I., Müller, S. & List, B. Kinetic resolution of homoaldols via catalytic asymmetric transacetalization. J. Am. Chem. Soc. 132, 17370–17373 (2010)
Klussmann, M. et al. Synthesis of TRIP and analysis of phosphate salt impurities. Synlett 2189–2192 (2010)
Wall, M. E., Eddy, C. R. & Serota, S. Steroidal sapogenins. XIX. Stereochemistry of sapogenins and cholesterol at carbon 20. J. Am. Chem. Soc. 76, 2849–2850 (1954)
Shapiro, N. D., Rauniyar, V., Hamilton, G. L., Wu, J. & Toste, F. D. Asymmetric additions to dienes catalysed by a dithiophosphoric acid. Nature 470, 245–249 (2011)
Mayer, S. & List, B. Asymmetric counteranion-directed catalysis. Angew. Chem. Int. Edn 45, 4193–4195 (2006)
Hamilton, G. L., Kang, E. J., Mba, M. & Toste, F. D. A powerful chiral counterion strategy for asymmetric transition metal catalysis. Science 317, 496–499 (2007)
Mukherjee, S. & List, B. Chiral counteranions in asymmetric transition-metal catalysis: highly enantioselective Pd/Brønsted acid-catalyzed direct α-allylation of aldehydes. J. Am. Chem. Soc. 129, 11336–11337 (2007)
Acknowledgements
We thank A. Dreier and R. Goddard for crystal structure analysis of catalyst 6a. S. Vellalath and S. Müller are acknowledged for donating several previously described catalysts, and N. Wippich and S. Dehn for technical assistance. We gratefully acknowledge support from the Max Planck Sociey and the European Research Council.
Author information
Authors and Affiliations
Contributions
I.Č. and B.L. jointly designed and developed C2-symmetric imidodiphosphoric acids, developed the spiroacetalization reaction, and wrote the manuscript. I.Č. conducted the laboratory experiments. B.L. initiated and oversaw the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-4, Supplementary Tables 1-6, Supplementary Methods, Supplementary References and Supplementary Data. The figures in the Supplementary Data show synthetic routes used and crystal structure of the catalyst 6a with probability ellipsoids. (PDF 4230 kb)
Supplementary Data
This file contains the crystallographic information for catalyst 6a. (ZIP 17 kb)
Rights and permissions
About this article
Cite this article
Čorić, I., List, B. Asymmetric spiroacetalization catalysed by confined Brønsted acids. Nature 483, 315–319 (2012). https://doi.org/10.1038/nature10932
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10932
This article is cited by
-
Privileged Brønsted acid organocatalysis
Nature Catalysis (2024)
-
Ratcheting synthesis
Nature Reviews Chemistry (2023)
-
Enantiodivergent epoxidation of alkenes with a photoswitchable phosphate manganese-salen complex
Nature Synthesis (2022)
-
Organocatalytic stereoselective cationic polymerization of vinyl ethers by employing a confined brønsted acid as the catalyst
Science China Chemistry (2022)
-
Asymmetric dearomatization catalysed by chiral Brønsted acids via activation of ynamides
Nature Chemistry (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.