Abstract
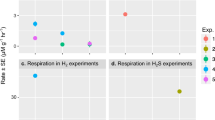
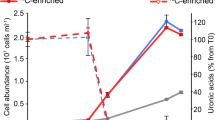
The discovery of deep-sea hydrothermal vents in 1977 revolutionized our understanding of the energy sources that fuel primary productivity on Earth. Hydrothermal vent ecosystems are dominated by animals that live in symbiosis with chemosynthetic bacteria. So far, only two energy sources have been shown to power chemosynthetic symbioses: reduced sulphur compounds and methane. Using metagenome sequencing, single-gene fluorescence in situ hybridization, immunohistochemistry, shipboard incubations and in situ mass spectrometry, we show here that the symbionts of the hydrothermal vent mussel Bathymodiolus from the Mid-Atlantic Ridge use hydrogen to power primary production. In addition, we show that the symbionts of Bathymodiolus mussels from Pacific vents have hupL, the key gene for hydrogen oxidation. Furthermore, the symbionts of other vent animals such as the tubeworm Riftia pachyptila and the shrimp Rimicaris exoculata also have hupL. We propose that the ability to use hydrogen as an energy source is widespread in hydrothermal vent symbioses, particularly at sites where hydrogen is abundant.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Accession codes
Data deposits
All hupL sequences have been deposited at NCBI under accession numbers FR851255–FR851274. The sequences that make up the genome fragment and the RAST annotation can be found at NCBI under project identification 65421 (accession numbers CAEB01000001–CAEB01000078).
References
Corliss, J. B. et al. Submarine thermal springs in the Galapagos Rift. Science 203, 1073–1083 (1979)
Cavanaugh, C. M., Gardiner, S. L., Jones, M. L., Jannasch, H. W. & Waterbury, J. B. Prokaryotic cells in the hydrothermal vent tube worm Riftia pachyptila Jones: possible chemoautotrophic symbionts. Science 213, 340–342 (1981)
Felbeck, H. Chemoautotrophic potential of the hydrothermal vent tube worm, Riftia pachyptila Jones (Vestimentifera). Science 213, 336–338 (1981)
Childress, J. J. et al. A methanotrophic marine molluscan (Bivalvia, Mytilidae) symbiosis: mussels fueled by gas. Science 233, 1306–1308 (1986)
Cavanaugh, C. M., Levering, P. R., Maki, J. S., Mitchell, R. & Lidstrom, M. E. Symbiosis of methylotrophic bacteria and deep-sea mussels. Nature 325, 346–348 (1987)
Dubilier, N., Bergin, C. & Lott, C. Symbiotic diversity in marine animals: the art of harnessing chemosynthesis. Nature Rev. Microbiol. 6, 725–740 (2008)
Tivey, M. K. Generation of seafloor hydrothermal vent fluids and associated mineral deposits. Oceanography (Wash. D.C.) 20, 50–65 (2007)
Fisher, C. R., Takai, K. & Le Bris, N. Hydrothermal vent ecosystems. Oceanography (Wash. D.C.) 20, 14–23 (2007)
Takai, K., Nakagawa, S., Reysenbach, A.-L. & Hock, J. In Back-Arc Spreading Systems—Geological, Biological, Chemical, and Physical Interactions (eds Christie, D. M. et al.) 185–213 (American Geophysical Union, 2006)
Perner, M. et al. The influence of ultramafic rocks on microbial communities at the Logatchev hydrothermal field, located 15° N on the Mid-Atlantic Ridge. FEMS Microbiol. Ecol. 61, 97–109 (2007)
Schmidt, K., Koschinsky, A., Garbe-Schönberg, D., de Carvalho, L. M. & Seifert, R. Geochemistry of hydrothermal fluids from the ultramafic-hosted Logatchev hydrothermal field, 15° N on the Mid-Atlantic Ridge: temporal and spatial investigation. Chem. Geol. 242, 1–21 (2007)
Amend, J. P. & Shock, E. L. Energetics of overall metabolic reactions of thermophilic and hyperthermophilic Archaea and Bacteria. FEMS Microbiol. Rev. 25, 175–243 (2001)
Gebruk, A. V., Chevaldonné, P., Shank, T., Lutz, R. A. & Vrijenhoek, R. C. Deep-sea hydrothermal vent communities of the Logatchev area (14° 45′ N, Mid-Atlantic Ridge): diverse biotopes and high biomass. J. Mar. Biol. Assoc. UK 80, 383–393 (2000)
Duperron, S. et al. A dual symbiosis shared by two mussel species, Bathymodiolus azoricus and Bathymodiolus puteoserpentis (Bivalvia: Mytilidae), from hydrothermal vents along the northern Mid-Atlantic Ridge. Environ. Microbiol. 8, 1441–1447 (2006)
Petersen, J. M. & Dubilier, N. Methanotrophic symbioses in marine invertebrates. Environ. Microbiol. Rep. 1, 319–335 (2009)
Wendeberg, A., Zielinski, F. U., Borowski, C. & Dubilier, N. Expression patterns of mRNAs for methanotrophy and thiotrophy in symbionts of the hydrothermal vent mussel Bathymodiolus puteoserpentis . ISME J. 10.1038/ismej.2011.81 (7 July 2011)
Vignais, P. M. & Billoud, B. Occurrence, classification, and biological function of hydrogenases: an overview. Chem. Rev. 107, 4206–4272 (2007)
Bernhard, M., Schwartz, E., Rietdorf, J. & Friedrich, B. The Alcaligenes eutrophus membrane-bound hydrogenase gene locus encodes functions involved in maturation and electron transport coupling. J. Bacteriol. 178, 4522–4529 (1996)
Meyer, O. & Schlegel, H. G. Reisolation of the carbon monoxide utilizing hydrogen bacterium Pseudomonas carboxydovorans (Kistner) comb. nov. Arch. Microbiol. 118, 35–43 (1978)
Schwartz, E. & Friedrich, B. in The Prokaryotes: A Handbook on the Biology of Bacteria (eds Dworkin, M. et al.) Vol. 2, 496–563 (Springer, 2006)
Nelson, D. C., Hagan, K. D. & Edwards, D. B. The gill symbiont of the hydrothermal vent mussel Bathymodiolus thermophilus is a psychrophilic, chemoautotrophic, sulfur bacterium. Mar. Biol. 121, 487–495 (1995)
Haase, K. M. et al. Diking, young volcanism and diffuse hydrothermal activity on the southern Mid-Atlantic Ridge: the Lilliput field at 9° 33′ S. Mar. Geol. 266, 52–64 (2009)
Haase, K. M. et al. Young volcanism and related hydrothermal activity at 5° S on the slow-spreading southern Mid-Atlantic Ridge. Geochem. Geophys. Geosys. 8, Q11002 (2007)
Friedrich, B. & Schwartz, E. Molecular biology of hydrogen utilization in aerobic chemolithotrophs. Annu. Rev. Microbiol. 47, 351–383 (1993)
Zielinski, F. U. et al. Widespread occurrence of an intranuclear bacterial parasite in vent and seep bathymodiolin mussels. Environ. Microbiol. 11, 1150–1167 (2009)
Ohmura, N., Sasaki, K., Matsumoto, N. & Saiki, H. Anaerobic respiration using Fe3+, S0, and H2 in the chemolithoautotrophic bacterium Acidithiobacillus ferrooxidans . J. Bacteriol. 184, 2081–2087 (2002)
Imhoff, J. F., Hiraishi, A. & Sühling, J. in Bergey’s Manual of Systematic Bacteriology (eds Brenner, D. J. et al.) Vol. 2, part A, 119–132 (Springer, 2005)
DiSpirito, A. A., Kunz, R. C., Choi, D.-W. & Zahn, J. A. in Respiration in Archaea and Bacteria. (ed. Zannoni, D. ) Vol. 2, 149–168 (Springer, 2004)
Olson, J. W. & Maier, R. J. Molecular hydrogen as an energy source for Helicobacter pylori . Science 298, 1788–1790 (2002)
Bowien, B. & Schlegel, H. G. Physiology and biochemistry of aerobic hydrogen-oxidizing bacteria. Annu. Rev. Microbiol. 35, 405–452 (1981)
Moraru, C., Lam, P., Fuchs, B. M., Kuypers, M. M. M. & Amann, R. GeneFISH—an in situ technique for linking gene presence and cell identity in environmental microorganisms. Environ. Microbiol. 12, 3057–3073 (2010)
Constant, P., Piossant, L. & Villemur, R. Tropospheric H2 budget and the response of its soil uptake under the changing environment. Sci. Total Environ. 407, 1809–1823 (2009)
Tromp, T., Shia, R.-L., Allen, M., Eiler, J. M. & Yung, Y. L. Potential environmental impact of a hydrogen economy on the stratosphere. Science 300, 1740–1742 (2003)
Perner, M., Petersen, J. M., Zielinski, F., Gennerich, H. H. & Seifert, R. Geochemical constraints on the diversity and activity of H2-oxidizing microorganisms in diffuse hydrothermal fluids from a basalt- and an ultramafic-hosted vent. FEMS Microbiol. Ecol. 74, 55–71 (2010)
Punshon, S., Moore, R. M. & Xie, H. Net loss rates and distribution of molecular hydrogen (H2) in mid-latitude coastal waters. Mar. Chem. 105, 129–139 (2007)
Welhan, J. A. & Craig, H. in Hydrothermal Processes at Seafloor Spreading Centers (eds Rona, P. A. et al.) 391–410 (Plenum, 1983)
Lilley, M. D., DeAngelis, M. A. & Gordon, L. I. CH4, H2, CO and N2O in submarine hydrothermal vent waters. Nature 300, 48–50 (1982)
Hügler, M., Petersen, J. M., Dubilier, N., Imhoff, J. F. & Sievert, S. M. Pathways of carbon and energy metabolism of the epibiotic community associated with the deep-sea hydrothermal vent shrimp Rimicaris exoculata . PLoS ONE 6, e16018 (2011)
Zhou, J. Z., Bruns, M. A. & Tiedje, J. M. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 62, 316–322 (1996)
Csáki, R., Hanczár, T., Bodrossy, L., Murrell, J. C. & Kovács, K. L. Molecular characterization of structural genes coding for a membrane bound hydrogenase in Methylococcus capsulatus (Bath). FEMS Microbiol. Lett. 205, 203–207 (2001)
Petersen, J. M. et al. Dual symbiosis of the vent shrimp Rimicaris exoculata with filamentous gamma- and epsilonproteobacteria at four Mid-Atlantic Ridge hydrothermal vent fields. Environ. Microbiol. 12, 2204–2218 (2010)
Ludwig, W. et al. ARB: a software environment for sequence data. Nucleic Acids Res. 32, 1363–1371 (2004)
Katoh, K., Asimenos, G. & Toh, H. Multiple alignment of DNA sequences with MAFFT. Methods Mol. Biol. 39–64. (2009)
Aziz, R. K. et al. The RAST server: Rapid annotations using subsystems technology. BMC Genomics 9, 75 (2008)
Moraru, C., Moraru, G., Fuchs, B. M. & Amann, R. Concepts and software for a rational design of polynucleotide probes. Environ. Microbiol. Rep. 3, 69–78 (2011)
Pernthaler, A., Pernthaler, J. & Amann, R. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 68, 3094–3101 (2002)
Wankel, S. D. et al. Influence of subsurface biosphere on geochemical fluxes from diffuse hydrothermal fluids. Nature Geosci. 4, 461–468 (2011)
Le Pennec, M. & Hily, A. Anatomie, structure et ultrastructure de la branchie d’un Mytilidae des sites hydrothermeaux du Pacifique oriental. Oceanol. Acta 7, 517–523 (1984)
Acknowledgements
We thank the chief scientists, and the captains and crews of the research vessels and remotely operated vehicles involved in sampling and analyses at sea. Thank you to D. Garbe-Schönberg, K. van der Heijden and J. Stecher for on-board sampling and analysis, and S. Duperron, M.-A. Cambon-Bonavita and M. Zbinden for providing samples. We acknowledge B. Friedrich and O. Lenz for the antiserum against the C. necator uptake hydrogenase, J. Milucka for help with western blots and T. Holler for culturing C. necator. S. Wetzel provided technical assistance. This work was supported by the German Science Foundation (DFG) Priority Program 1144 “From Mantle to Ocean: Energy-, Material- and Life Cycles at Spreading Axes” (publication number 60), the DFG Cluster of Excellence “The Ocean in the Earth System” at MARUM (Center for Marine Environmental Sciences), and the Max Planck Society.
Author information
Authors and Affiliations
Contributions
J.M.P., F.U.Z., T.P., R.S., C.B., D.F. and N.D. did the on-board experiments during the research cruises. F.U.Z. analysed the data from physiology experiments. J.M.P. amplified and sequenced hupL, analysed the genome data, did western blots and immunohistochemistry. C.M. and R.A. did the geneFISH. S.H., S.D.W. and P.R.G. did the in situ mass spectrometry and analysed the data. V.B. and E.P. did the genome sequencing and assembly. W.B. did the thermodynamic modelling. J.M.P., F.U.Z. and N.D. conceived the study and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
The file contains, Supplementary Materials and Methods, a Supplementary Discussion, Supplementary Figures 1-7 with legends, Supplementary Tables 1-7 and additional references. (PDF 3734 kb)
Rights and permissions
About this article
Cite this article
Petersen, J., Zielinski, F., Pape, T. et al. Hydrogen is an energy source for hydrothermal vent symbioses. Nature 476, 176–180 (2011). https://doi.org/10.1038/nature10325
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10325
This article is cited by
-
The genome of a vestimentiferan tubeworm (Ridgeia piscesae) provides insights into its adaptation to a deep-sea environment
BMC Genomics (2023)
-
Sulfur, sterol and trehalose metabolism in the deep-sea hydrocarbon seep tubeworm Lamellibrachia luymesi
BMC Genomics (2023)
-
Molecular hydrogen in seawater supports growth of diverse marine bacteria
Nature Microbiology (2023)
-
Facile engineering of Co3O4/Pr2O3 nanostructure for boosted oxygen evolution reaction
Applied Physics A (2023)
-
Global patterns of diversity and metabolism of microbial communities in deep-sea hydrothermal vent deposits
Microbiome (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.