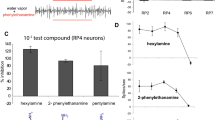
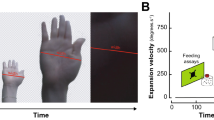
Abstract
Carbon dioxide (CO2) present in exhaled air is the most important sensory cue for female blood-feeding mosquitoes, causing activation of long-distance host-seeking flight, navigation towards the vertebrate host1 and, in the case of Aedes aegypti, increased sensitivity to skin odours2. The CO2 detection machinery is therefore an ideal target to disrupt host seeking. Here we use electrophysiological assays to identify a volatile odorant that causes an unusual, ultra-prolonged activation of CO2-detecting neurons in three major disease-transmitting mosquitoes: Anopheles gambiae, Culex quinquefasciatus and A. aegypti. Importantly, ultra-prolonged activation of these neurons severely compromises their ability subsequently to detect CO2 for several minutes. We also identify odours that strongly inhibit CO2-sensitive neurons as candidates for use in disruption of host-seeking behaviour, as well as an odour that evokes CO2-like activity and thus has potential use as a lure in trapping devices. Analysis of responses to panels of structurally related odours across the three mosquitoes and Drosophila, which have related CO2-receptor proteins, reveals a pattern of inhibition that is often conserved. We use video tracking in wind-tunnel experiments to demonstrate that the novel ultra-prolonged activators can completely disrupt CO2-mediated activation as well as source-finding behaviour in Aedes mosquitoes, even after the odour is no longer present. Lastly, semi-field studies demonstrate that use of ultra-prolonged activators disrupts CO2-mediated hut entry behaviour of Culex mosquitoes. The three classes of CO2-response-modifying odours offer powerful instruments for developing new generations of insect repellents and lures, which even in small quantities can interfere with the ability of mosquitoes to seek humans.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gillies, M. T. The role of carbon dioxide in host-finding by mosquitoes: a review. Bull. Entomol. Res. 70, 525–532 (1980)
Dekker, T., Geier, M. & Carde, R. T. Carbon dioxide instantly sensitizes female yellow fever mosquitoes to human skin odours. J. Exp. Biol. 208, 2963–2972 (2005)
Zwiebel, L. J. & Takken, W. Olfactory regulation of mosquito-host interactions. Insect Biochem. Mol. Biol. 34, 645–652 (2004)
Ditzen, M., Pellegrino, M. & Vosshall, L. B. Insect odorant receptors are molecular targets of the insect repellent DEET. Science 319, 1838–1842 (2008)
Syed, Z. & Leal, W. S. Mosquitoes smell and avoid the insect repellent DEET. Proc. Natl Acad. Sci. USA 105, 13598–13603 (2008)
Krajick, K. Keeping the bugs at bay. Science 313, 36–38 (2006)
Corbel, V. et al. Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent DEET. BMC Biol. 7, 47 (2009)
Reeder, N. L., Ganz, P. J., Carlson, J. R. & Saunders, C. W. Isolation of a DEET-insensitive mutant of Drosophila melanogaster (Diptera: Drosophilidae). J. Econ. Entomol. 94, 1584–1588 (2001)
Klun, J. A. et al. Comparative resistance of Anopheles albimanus and Aedes aegypti to N,N-diethyl-3-methylbenzamide (Deet) and 2-methylpiperidinyl-3-cyclohexen-1-carboxamide (AI3-37220) in laboratory human volunteer repellent assays. J. Med. Entomol. 41, 418–422 (2004)
Stanczyk, N. M., Brookfield, J. F., Ignell, R., Logan, J. G. & Field, L. M. Behavioral insensitivity to DEET in Aedes aegypti is a genetically determined trait residing in changes in sensillum function. Proc. Natl Acad. Sci. USA 107, 8575–8580 (2010)
Grant, A. J. & O’Connell, R. J. Electrophysiological responses from receptor neurons in mosquito maxillary palp sensilla. Ciba Found. Symp. 200, 233–253 (1996)
Zollner, G. E., Torr, S. J., Ammann, C. & Meixner, F. X. Dispersion of carbon dioxide plumes in African woodland: implications for host-finding by tsetse flies. Physiol. Entomol. 29, 381–394 (2004)
Carde, R. T. & Gibson, G. in Olfaction in Vector-Host Interactions Vol. 2 (eds Takken, W. & Knols, B. G. F. ) 115–141 (Wageningen Academic Publishers, 2010)
Robertson, H. M. & Kent, L. B. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J. Insect Sci. 9, 1–14 (2009)
Lu, T. et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae . Curr. Biol. 17, 1533–1544 (2007)
Jones, W. D., Cayirlioglu, P., Kadow, I. G. & Vosshall, L. B. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila . Nature 445, 86–90 (2007)
Kwon, J. Y., Dahanukar, A., Weiss, L. A. & Carlson, J. R. The molecular basis of CO2 reception in Drosophila . Proc. Natl Acad. Sci. USA 104, 3574–3578 (2007)
Syed, Z. & Leal, W. S. Maxillary palps are broad spectrum odorant detectors in Culex quinquefasciatus . Chem. Senses 32, 727–738 (2007)
Turner, S. L. & Ray, A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature 461, 277–281 (2009)
Zdobnov, E. M. et al. Comparative genome and proteome analysis of Anopheles gambiae and Drosophila melanogaster . Science 298, 149–159 (2002)
de Bruyne, M., Foster, K. & Carlson, J. Odor coding in the Drosophila antenna. Neuron 30, 537–552 (2001)
Hallem, E. A. & Carlson, J. R. Coding of odors by a receptor repertoire. Cell 125, 143–160 (2006)
Rostelien, T., Borg-Karlson, A. K., Faldt, J., Jacobsson, U. & Mustaparta, H. The plant sesquiterpene germacrene D specifically activates a major type of antennal receptor neuron of the tobacco budworm moth Heliothis virescens . Chem. Senses 25, 141–148 (2000)
Kaissling, K.-E., Meng, L. Z. & Bestmann, H.-J. Responses of bombykol receptor cells to (Z,E)-4,6-hexadecadiene and linalool. J. Comp. Physiol. A 165, 147–154 (1989)
Yao, C. A. & Carlson, J. R. Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila . J. Neurosci. 30, 4562–4572 (2010)
Mafra-Neto, A. & Carde, R. T. Fine-scale structure of pheromone plumes modulates upwind orientation of flying moths. Nature 369, 142–144 (1994)
Bhandawat, V., Maimon, G., Dickinson, M. H. & Wilson, R. I. Olfactory modulation of flight in Drosophila is sensitive, selective and rapid. J. Exp. Biol. 213, 3625–3635 (2010)
Bhandawat, V., Olsen, S. R., Gouwens, N. W., Schlief, M. L. & Wilson, R. I. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nature Neurosci. 10, 1474–1482 (2007)
Noosidum, A., Prabaripai, A., Chareonviriyaphap, T. & Chandrapatya, A. Excito-repellency properties of essential oils from Melaleuca leucadendron L. Litsea cubeba (Lour.) Persoon, and Litsea salicifolia (Nees) on Aedes aegypti (L.) mosquitoes. J. Vector Ecol. 33, 305–312 (2008)
Njiru, B. N., Mukabana, W. R., Takken, W. & Knols, B. G. Trapping of the malaria vector Anopheles gambiae with odour-baited MM-X traps in semi-field conditions in western Kenya. Malar. J. 5, 39 (2006)
Acknowledgements
We thank E. Lacey and S. McInally for help setting up behaviour experiments; the Malaria Group team members in ICIPE Mbita Point Research Station, Y. Afrane and G. Yan for logistical support; S. M. Boyle for help with Pharmacophores; K. Klingler for help with statistics; J. Perecko for building traps and excitorepellency chambers; A. Dahanukar and G. Tauxe for comments on the manuscript; W. Walton, P. Atkinson, P. Wirth and A. Khalon for mosquitoes. Part of this work was funded by a grant to A. Ray from the Bill & Melinda Gates Foundation through the Grand Challenges Exploration Initiative, and part supported by a grant to A. Ray, Award Number R01AI087785, from the NIAID (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or NIH.
Author information
Authors and Affiliations
Contributions
S.L.T. planned the electrophysiology experiments, performed the experiments, collected and analysed the data and helped write the paper. N.L. performed the wind tunnel, repellency, MalariaSphere and greenhouse behaviour experiments and analysed the data. T.G. performed one-choice behaviour experiments. J.G. helped plan MalariaSphere experiments. R.T.C. helped plan the behaviour experiments, and helped write the paper. A.R. helped plan the experiments, analysed the data, managed the project and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
A.R and S.L.T. are listed as inventors in a pending patent application, which is filed by the University of California, Riverside. A.R serves as consultant for a corporation that has obtained the license for this pending patent application from University of California, Riverside.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9 with legends and Supplementary Table 1. (PDF 9339 kb)
Supplementary Movie 1
The file movie contains a representative video of a mock-treated female A. aegypti mosquito navigating upwind and through a CO2 turbulent-plume releasing ring in real time. (MOV 847 kb)
Supplementary Movie 2
This movie file contains a representative video of a pretreated (ultra-prolonged odour blend, 10-2) female A. aegypti mosquito, unable to find upwind CO2 source in real time. For the remaining duration of the 300 sec assay the mosquito does not move after coming to rest on the glass wall. (MOV 3363 kb)
Supplementary Movie 3
This file movie contains a representative video of a pretreated (ultra-prolonged odour blend, 10-1) female A. aegypti mosquito, unable to activate upwind flight from the cage and find upwind CO2 source during the entire duration of the 300 sec assay (video speeded up). (MOV 2601 kb)
Rights and permissions
About this article
Cite this article
Turner, S., Li, N., Guda, T. et al. Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature 474, 87–91 (2011). https://doi.org/10.1038/nature10081
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10081
This article is cited by
-
Comparison of entomological impacts of two methods of intervention designed to control Anopheles gambiae s.l. via swarm killing in Western Burkina Faso
Scientific Reports (2022)
-
Putative ligand binding sites of two functionally characterized bark beetle odorant receptors
BMC Biology (2021)
-
Evaluating putative repellent ‘push’ and attractive ‘pull’ components for manipulating the odour orientation of host-seeking malaria vectors in the peri-domestic space
Parasites & Vectors (2021)
-
Mosquito Attractants
Journal of Chemical Ecology (2021)
-
Adaptive temporal processing of odor stimuli
Cell and Tissue Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.