Abstract
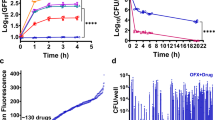
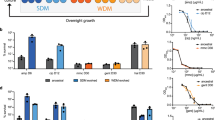
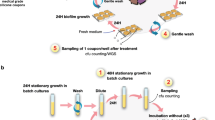
Bacterial persistence is a state in which a sub-population of dormant cells, or ‘persisters’, tolerates antibiotic treatment1,2,3,4. Bacterial persisters have been implicated in biofilms and in chronic and recurrent infections5,6,7. Despite this clinical relevance, there are currently no viable means for eradicating persisters. Here we show that specific metabolic stimuli enable the killing of both Gram-negative (Escherichia coli) and Gram-positive (Staphylococcus aureus) persisters with aminoglycosides. This potentiation is aminoglycoside-specific, it does not rely on growth resumption and it is effective in both aerobic and anaerobic conditions. It proceeds by the generation of a proton-motive force which facilitates aminoglycoside uptake. Our results demonstrate that persisters, although dormant, are primed for metabolite uptake, central metabolism and respiration. We show that aminoglycosides can be used in combination with specific metabolites to treat E. coli and S. aureus biofilms. Furthermore, we demonstrate that this approach can improve the treatment of chronic infections in a mouse urinary tract infection model. This work establishes a strategy for eradicating bacterial persisters that is based on metabolism, and highlights the importance of the metabolic environment to antibiotic treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Balaban, N. Q., Merrin, J., Chait, R., Kowalik, L. & Leibler, S. Bacterial persistence as a phenotypic switch. Science 305, 1622–1625 (2004)
Gefen, O., Gabay, C., Mumcuoglu, M., Engel, G. & Balaban, N. Q. Single-cell protein induction dynamics reveals a period of vulnerability to antibiotics in persister bacteria. Proc. Natl Acad. Sci. USA 105, 6145–6149 (2008)
Gefen, O. & Balaban, N. Q. The importance of being persistent: heterogeneity of bacterial populations under antibiotic stress. FEMS Microbiol. Rev. 33, 704–717 (2009)
Lewis, K. Persister cells, dormancy and infectious disease. Nature Rev. Microbiol. 5, 48–56 (2007)
Smith, P. A. & Romesberg, F. E. Combating bacteria and drug resistance by inhibiting mechanisms of persistence and adaptation. Nature Chem. Biol. 3, 549–556 (2007)
Levin, B. R. & Rozen, D. E. Non-inherited antibiotic resistance. Nature Rev. Microbiol. 4, 556–562 (2006)
Dhar, N. & McKinney, J. D. Microbial phenotypic heterogeneity and antibiotic tolerance. Curr. Opin. Microbiol. 10, 30–38 (2007)
Shah, D. et al. Persisters: a distinct physiological state of E. coli . BMC Microbiol. 6, 53 (2006)
Vakulenko, S. B. & Mobashery, S. Versatility of aminoglycosides and prospects for their future. Clin. Microbiol. Rev. 16, 430–450 (2003)
Magnet, S. & Blanchard, J. S. Molecular insights into aminoglycoside action and resistance. Chem. Rev. 105, 477–498 (2005)
Davis, B. D. Mechanism of bactericidal action of aminoglycosides. Microbiol. Rev. 51, 341–350 (1987)
Weisblum, B. & Davies, J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol. Rev. 32, 493–528 (1968)
Kohanski, M. A., Dwyer, D. J., Wierzbowski, J., Cottarel, G. & Collins, J. J. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell 135, 679–690 (2008)
Keren, I., Shah, D., Spoering, A., Kaldalu, N. & Lewis, K. Specialized persister cells and the mechanism of multidrug tolerance in Escherichia coli . J. Bacteriol. 186, 8172–8180 (2004)
Spoering, A. L. & Lewis, K. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing by antimicrobials. J. Bacteriol. 183, 6746–6751 (2001)
Taber, H. W., Mueller, J. P., Miller, P. F. & Arrow, A. S. Bacterial uptake of aminoglycoside antibiotics. Microbiol. Rev. 51, 439–457 (1987)
Bryan, L. E. & Kwan, S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob. Agents Chemother. 23, 835–845 (1983)
Hill, S., Viollet, S., Smith, A. T. & Anthony, C. Roles for enteric d-type cytochrome oxidase in N2 fixation and microaerobiosis. J. Bacteriol. 172, 2071–2078 (1990)
Govantes, F., Albrecht, J. A. & Gunsalus, R. P. Oxygen regulation of the Escherichia coli cytochrome d oxidase (cydAB) operon: roles of multiple promoters and the Fnr-1 and Fnr-2 binding sites. Mol. Microbiol. 37, 1456–1469 (2000)
Walters, M. C., III, Roe, F., Bugnicourt, A., Franklin, M. J. & Stewart, P. S. Contributions of antibiotic penetration, oxygen limitation, and low metabolic activity to tolerance of Pseudomonas aeruginosa biofilms to ciprofloxacin and tobramycin. Antimicrob. Agents Chemother. 47, 317–323 (2003)
Mates, S. M. et al. Membrane potential and gentamicin uptake in Staphylococcus aureus . Proc. Natl Acad. Sci. USA 79, 6693–6697 (1982)
Fraimow, H. S., Greenman, J. B., Leviton, I. M., Dougherty, T. J. & Miller, M. H. Tobramycin uptake in Escherichia coli is driven by either electrical potential or ATP. J. Bacteriol. 173, 2800–2808 (1991)
Dwyer, D. J., Kohanski, M. A., Hayete, B. & Collins, J. J. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli . Mol. Syst. Biol. 3, 91 (2007)
Kohanski, M. A., Dwyer, D. J., Hayete, B., Lawrence, C. A. & Collins, J. J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130, 797–810 (2007)
Keren, I., Kaldalu, N., Spoering, A., Wang, Y. & Lewis, K. Persister cells and tolerance to antimicrobials. FEMS Microbiol. Lett. 230, 13–18 (2004)
Hansen, S., Lewis, K. & Vulic, M. Role of global regulators and nucleotide metabolism in antibiotic tolerance in Escherichia coli . Antimicrob. Agents Chemother. 52, 2718–2726 (2008)
Sandoval, R., Leiser, J. & Molitoris, B. A. Aminoglycoside antibiotics traffic to the Golgi complex in LLC-PK1 cells. J. Am. Soc. Nephrol. 9, 167–174 (1998)
Lu, T. K. & Collins, J. J. Dispersing biofilms with engineered enzymatic bacteriophage. Proc. Natl Acad. Sci. USA 104, 11197–11202 (2007)
Majerczyk, C. D. et al. Direct targets of CodY in Staphylococcus aureus . J. Bacteriol. 192, 2861–2877 (2010)
Irizarry, R. A. et al. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31, e15 (2003)
Acknowledgements
We thank A. Slee and T. Murphy from ViviSource Laboratories for assistance with the in vivo mouse studies and T. K. Lu for guidance with the biofilm experiments. This work was supported by the NIH Director’s Pioneer Award Program and the Howard Hughes Medical Institute.
Author information
Authors and Affiliations
Contributions
All authors designed the study, analysed results and wrote the manuscript. Experiments were performed by K.R.A. and M.P.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Results, additional references, Supplementary Figures 1-27 with legends and Supplementary Tables 1-3. (PDF 1889 kb)
Rights and permissions
About this article
Cite this article
Allison, K., Brynildsen, M. & Collins, J. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473, 216–220 (2011). https://doi.org/10.1038/nature10069
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10069
This article is cited by
-
Single-cell massively-parallel multiplexed microbial sequencing (M3-seq) identifies rare bacterial populations and profiles phage infection
Nature Microbiology (2023)
-
A mini-review: environmental and metabolic factors affecting aminoglycoside efficacy
World Journal of Microbiology and Biotechnology (2023)
-
The Potential Role of Persister Cells in Urinary Tract Infections
Current Urology Reports (2023)
-
Identification of metabolite extraction method for targeted exploration of antimicrobial resistance associated metabolites of Klebsiella pneumoniae
Scientific Reports (2022)
-
Potentiating hypoxic microenvironment for antibiotic activation by photodynamic therapy to combat bacterial biofilm infections
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.