Abstract
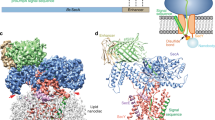
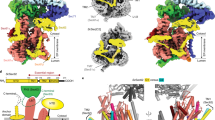
Protein translocation across the bacterial membrane, mediated by the secretory translocon SecYEG and the SecA ATPase1,2,3,4, is enhanced by proton motive force5,6 and membrane-integrated SecDF7,8,9, which associates with SecYEG. The role of SecDF has remained unclear, although it is proposed to function in later stages of translocation as well as in membrane protein biogenesis4,10,11,12,13. Here, we determined the crystal structure of Thermus thermophilus SecDF at 3.3 Å resolution, revealing a pseudo-symmetrical, 12-helix transmembrane domain belonging to the RND superfamily and two major periplasmic domains, P1 and P4. Higher-resolution analysis of the periplasmic domains suggested that P1, which binds an unfolded protein, undergoes functionally important conformational changes. In vitro analyses identified an ATP-independent step of protein translocation that requires both SecDF and proton motive force. Electrophysiological analyses revealed that SecDF conducts protons in a manner dependent on pH and the presence of an unfolded protein, with conserved Asp and Arg residues at the transmembrane interface between SecD and SecF playing essential roles in the movements of protons and preproteins. Therefore, we propose that SecDF functions as a membrane-integrated chaperone, powered by proton motive force, to achieve ATP-independent protein translocation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Primary accessions
Biological Magnetic Resonance Data Bank
Protein Data Bank
Data deposits
The coordinates and structure factors have been deposited in the Protein Data Bank under the accession codes 3AQP for the entire TtSecDF protein and 3AQO for the P1 domain. The PDB and BMRB codes for the deposited P4 domain are 2RRN and 11426 respectively.
References
van den Berg, B. et al. X-ray structure of a protein-conducting channel. Nature 427, 36–44 (2004)
Zimmer, J., Nam, Y. & Rapoport, T. A. Structure of a complex of the ATPase SecA and the protein-translocation channel. Nature 455, 936–943 (2008)
Tsukazaki, T. et al. Conformational transition of Sec machinery inferred from bacterial SecYE structures. Nature 455, 988–991 (2008)
du Plessis, D. J., Nouwen, N. & Driessen, A. J. The Sec translocase. Biochim. Biophys. Acta 1808, 851–865 (2011)
Driessen, A. J. & Wickner, W. Proton transfer is rate-limiting for translocation of precursor proteins by the Escherichia coli translocase. Proc. Natl Acad. Sci. USA 88, 2471–2475 (1991)
Shiozuka, K., Tani, K., Mizushima, S. & Tokuda, H. The proton motive force lowers the level of ATP required for the in vitro translocation of a secretory protein in Escherichia coli . J. Biol. Chem. 265, 18843–18847 (1990)
Pogliano, J. A. & Beckwith, J. SecD and SecF facilitate protein export in Escherichia coli . EMBO J. 13, 554–561 (1994)
Sagara, K., Matsuyama, S. & Mizushima, S. SecF stabilizes SecD and SecY, components of the protein translocation machinery of the Escherichia coli cytoplasmic membrane. J. Bacteriol. 176, 4111–4116 (1994)
Hand, N. J., Klein, R., Laskewitz, A. & Pohlschroder, M. Archaeal and bacterial SecD and SecF homologs exhibit striking structural and functional conservation. J. Bacteriol. 188, 1251–1259 (2006)
Matsuyama, S., Fujita, Y. & Mizushima, S. SecD is involved in the release of translocated secretory proteins from the cytoplasmic membrane of Escherichia coli . EMBO J. 12, 265–270 (1993)
Economou, A., Pogliano, J. A., Beckwith, J., Oliver, D. B. & Wickner, W. SecA membrane cycling at SecYEG is driven by distinct ATP binding and hydrolysis events and is regulated by SecD and SecF. Cell 83, 1171–1181 (1995)
Arkowitz, R. A. & Wickner, W. SecD and SecF are required for the proton electrochemical gradient stimulation of preprotein translocation. EMBO J. 13, 954–963 (1994)
Duong, F. & Wickner, W. The SecDFyajC domain of preprotein translocase controls preprotein movement by regulating SecA membrane cycling. EMBO J. 16, 4871–4879 (1997)
Nouwen, N., Piwowarek, M., Berrelkamp, G. & Driessen, A. J. The large first periplasmic loop of SecD and SecF plays an important role in SecDF functioning. J. Bacteriol. 187, 5857–5860 (2005)
Pogliano, K. J. & Beckwith, J. Genetic and molecular characterization of the Escherichia coli secD operon and its products. J. Bacteriol. 176, 804–814 (1994)
Tseng, T. T. et al. The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J. Mol. Microbiol. Biotechnol. 1, 107–125 (1999)
Tsukazaki, T. et al. Purification, crystallization and preliminary X-ray diffraction of SecDF, a translocon-associated membrane protein, from Thermus thermophilus. Acta Crystallogr. F 62, 376–380 (2006)
Uchida, K., Mori, H. & Mizushima, S. Stepwise movement of preproteins in the process of translocation across the cytoplasmic membrane of Escherichia coli . J. Biol. Chem. 270, 30862–30868 (1995)
Schiebel, E., Driessen, A. J., Hartl, F. U. & Wickner, W. ΔμH+ and ATP function at different steps of the catalytic cycle of preprotein translocase. Cell 64, 927–939 (1991)
Murakami, S., Nakashima, R., Yamashita, E., Matsumoto, T. & Yamaguchi, A. Crystal structures of a multidrug transporter reveal a functionally rotating mechanism. Nature 443, 173–179 (2006)
Seeger, M. A., von Ballmoos, C., Verrey, F. & Pos, K. M. Crucial role of Asp408 in the proton translocation pathway of multidrug transporter AcrB: evidence from site-directed mutagenesis and carbodiimide labeling. Biochemistry 48, 5801–5812 (2009)
Häse, C. C. & Barquera, B. Role of sodium bioenergetics in Vibrio cholerae . Biochim. Biophys. Acta 1505, 169–178 (2001)
Sasaki, M., Takagi, M. & Okamura, Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science 312, 589–592 (2006)
Hattori, M. et al. Mg2+-dependent gating of bacterial MgtE channel underlies Mg2+ homeostasis. EMBO J. 28, 3602–3612 (2009)
Vassylyev, D. G. et al. Structural basis for substrate loading in bacterial RNA polymerase. Nature 448, 163–168 (2007)
Inaba, K. et al. Crystal structure of the DsbB-DsbA complex reveals a mechanism of disulfide bond generation. Cell 127, 789–801 (2006)
Matsuo, E., Mori, H., Shimoike, T. & Ito, K. Syd, a SecY-interacting protein, excludes SecA from the SecYE complex with an altered SecY24 subunit. J. Biol. Chem. 273, 18835–18840 (1998)
Acknowledgements
We thank Y. Akiyama, R. Suno, Y. Morimoto, T. Minamino, K. Namba, K. Inaba, M. Hattori and H. Nishimasu for suggestions; T. Sakamoto and A. Kurabayashi for assistance with sample preparation; R. Yamasaki, M. Sano, K. Mochizuki, K. Yoshikaie, K. Imayoshi and T. Adachi for technical support; M. Homma and S. Kojima for providing the Vibrio genomic DNA; the beamline staff members at BL41XU of SPring-8 (Hyogo, Japan) and at NW12 of KEK PF-AR (Tsukuba, Japan) for technical help during data collection and M. Ibba for comments on our manuscript. This work was supported by a Grant-in-Aid for Scientific Research (S) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) to O.N., by a CREST grant from JST to K.I., by a BIRD grant from JST to H.M. and R.I., by a grant for the National Project on Protein Structural and Functional Analyses to O.N., by NIH grants to D.G.V. and by grants from MEXT to T.Tsukazaki, H.M., R.I., S.F. and K.I.
Author information
Authors and Affiliations
Contributions
T.Tsukazaki performed the structural determination and the biochemical experiments with SecDF. H.M. performed the functional analyses of SecDF. Y.E. solved the crystal structure of the SecDF P1 domain and assisted with the functional analysis of SecDF. R.I., S.F., D.G.V. and O.N. assisted with the structural determination. A.D.M. performed patch clamp and pH fluorescence experiments. T.Tanaka and T.K. solved the structure of the P4 domain by NMR. A.P. and D.G.V. assisted with the crystallization and data collection of SecDF. All authors discussed the results and commented on the manuscript. O.N. and K.I. supervised the work and wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion, Supplementary Methods, Supplementary References, Supplementary Figures 1- 9 with legends and Supplementary Tables 1-2. (PDF 1906 kb)
Rights and permissions
About this article
Cite this article
Tsukazaki, T., Mori, H., Echizen, Y. et al. Structure and function of a membrane component SecDF that enhances protein export. Nature 474, 235–238 (2011). https://doi.org/10.1038/nature09980
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09980
This article is cited by
-
Cellular dynamics of the SecA ATPase at the single molecule level
Scientific Reports (2021)
-
Structural Basis of the Sec Translocon and YidC Revealed Through X-ray Crystallography
The Protein Journal (2019)
-
Genetic Analysis of Protein Translocation
The Protein Journal (2019)
-
Protein Transport Across the Bacterial Plasma Membrane by the Sec Pathway
The Protein Journal (2019)
-
Comprehensive subcellular topologies of polypeptides in Streptomyces
Microbial Cell Factories (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.