Abstract
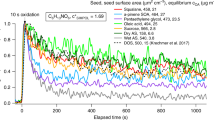
Secondary organic aerosol (SOA) particles are formed in the atmosphere from condensable oxidation products of anthropogenic and biogenic volatile organic compounds (VOCs)1,2,3,4,5,6,7. On a global scale, biogenic VOCs account for about 90% of VOC emissions1,8 and of SOA formation (90 billion kilograms of carbon per year)1,2,3,4. SOA particles can scatter radiation and act as cloud condensation or ice nuclei, and thereby influence the Earth’s radiation balance and climate1,2,5,9,10. They consist of a myriad of different compounds with varying physicochemical properties, and little information is available on the phase state of SOA particles. Gas–particle partitioning models usually assume that SOA particles are liquid1,5,11, but here we present experimental evidence that they can be solid under ambient conditions. We investigated biogenic SOA particles formed from oxidation products of VOCs in plant chamber experiments and in boreal forests within a few hours after atmospheric nucleation events. On the basis of observed particle bouncing in an aerosol impactor and of electron microscopy we conclude that biogenic SOA particles can adopt an amorphous solid—most probably glassy—state. This amorphous solid state should provoke a rethinking of SOA processes because it may influence the partitioning of semi-volatile compounds, reduce the rate of heterogeneous chemical reactions, affect the particles’ ability to accommodate water and act as cloud condensation or ice nuclei, and change the atmospheric lifetime of the particles12,13,14,15. Thus, the results of this study challenge traditional views of the kinetics and thermodynamics of SOA formation and transformation in the atmosphere and their implications for air quality and climate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Hallquist, M. et al. The formation, properties and impact of secondary organic aerosol: current and emerging issues. Atmos. Chem. Phys. 9, 5155–5236 (2009)
Kanakidou, M. et al. Organic aerosol and global climate modelling: a review. Atmos. Chem. Phys. 5, 1053–1123 (2005)
Kavouras, G. I., Mihalopoulos, N. & Stephanou, E. G. Formation of atmospheric particles from organic acids produced by forest. Nature 395, 683–686 (1998)
Claeys, M. et al. Formation of secondary organic aerosols through photooxidation of isoprene. Science 303, 1173–1176 (2004)
Jimenez, J. L. et al. Evolution of organic aerosols in the atmosphere. Science 326, 1525–1529 (2009)
Laaksonen, A. et al. The role of VOC oxidation products in continental new particle formation. Atmos. Chem. Phys. 8, 2657–2665 (2008)
Tunved, P. et al. High natural aerosol loading over boreal forests. Science 312, 261–263 (2006)
Guenther, A. et al. A global model of natural volatile organic compound emissions. J. Geophys. Res. 100 (D5). 8873–8892 (1995)
Fuzzi, S. et al. Critical assessment of the current state of scientific knowledge, terminology, and research needs concerning the role of organic aerosols in the atmosphere, climate, and global change. Atmos. Chem. Phys. 6, 2017–2038 (2006)
Intergovernmental Panel on Climate Change (IPCC). Climate Change 2007: The Physical Science Basis Ch. 2 161–177 Ch. 7 556–564 (Cambridge University Press, 2007)
Pankow, J. F. An absorption model of the gas/aerosol partitioning involved in the formation of secondary organic aerosol. Atmos. Environ. 28, 189–193 (1994)
Zahardis, J. & Petrucci, G. A. The oleic acid-ozone heterogeneous reaction system: products, kinetics, secondary chemistry, and atmospheric implications of a model system—a review. Atmos. Chem. Phys. 7, 1237–1274 (2007)
Zobrist, B., Marcolli, C., Pedernera, D. A. & Koop, T. Do atmospheric aerosols form glasses? Atmos. Chem. Phys. 8, 5221–5244 (2008)
Murray, B. J. Inhibition of ice crystallisation in highly viscous aqueous organic acid droplets. Atmos. Chem. Phys. 8, 5423–5433 (2008)
Mikhailov, E., Vlasenko, S., Martin, S. T., Koop, T. & Pöschl, U. Amorphous and crystalline aerosol particles interacting with water vapor: conceptual framework and experimental evidence for restructuring, phase transitions and kinetic limitations. Atmos. Chem. Phys. 9, 9491–9522 (2009)
Joutsensaari, J. et al. Nanoparticle formation by ozonolysis of inducible plant volatiles. Atmos. Chem. Phys. 5, 1489–1495 (2005)
Mentel, Th, F. et al. Photochemical production of aerosols from real plant emissions. Atmos. Chem. Phys. 9, 4387–4406 (2009)
Dahneke, B. Capture of aerosol particles by surfaces. J. Colloid Interf. Sci. 37, 342–347 (1971)
Stein, S. W., Turpin, J. B., Cai, X., Huang, P. F. & McMurry, P. H. Measurements of relative humidity dependent bounce and density for atmospheric particles using DMA-impactor technique. Atmos. Environ. 28, 1739–1746 (1994)
Marcolli, C., Luo, B. P. & Peter, T. Mixing of the organic aerosol fractions: liquids as the thermodynamically stable phases. J. Phys. Chem. A 108, 2216–2224 (2004)
Kalberer, M. et al. Identification of polymers as major components of atmospheric organic aerosols. Science 303, 1659–1662 (2004)
Hari, P. & Kulmala, M. Station for measuring ecosystem-atmosphere relations (SMEAR II). Boreal Environ. Res. 10, 315–322 (2005)
Pankow, J. F. Review and comparative analysis of the theories of partitioning between the gas and aerosol particulate phases in the atmosphere. Atmos. Environ. 21, 2275–2283 (1987)
Ehn, M. et al. Hygroscopic properties of ultrafine particles in the boreal forest: diurnal variation, solubility and the influence of sulfuric acid. Atmos. Chem. Phys. 7, 211–222 (2007)
Hämeri, K. & Väkevä, M. Ultrafine aerosol particle hygroscopicity and volatility in boreal forest. Rep. Ser. Aerosol Sci. 47, 47–59 (2000)
Bahreini, R. et al. Measurements of secondary organic aerosol from oxidation of cycloalkanes, terpenes, and m-xylene using an aerodyne aerosol mass spectrometer. Environ. Sci. Technol. 39, 5674–5688 (2005)
Maria, S. F., Russell, L. M., Gilles, M. K. & Myneni, S. C. B. Organic aerosol growth mechanisms and their climate-forcing implications. Science 306, 1921–1924 (2004)
Parker, R. & Ring, S. G. Diffusion in maltose-water mixtures at temperatures close to the glass transition. Carbohydr. Res. 273, 147–155 (1995)
Shiraiwa, M., Pfrang, C. & Pöschl, U. Kinetic multi-layer model of aerosol surface and bulk chemistry (KM-SUB): the influence of interfacial transport and bulk diffusion on the oxidation of oleic acid by ozone. Atmos. Chem. Phys. 10, 3673–3691 (2010)
Acknowledgements
We acknowledge support by the Academy of Finland (decision numbers 110763, 111543, 131019, 218115 and Centre of Excellence Programme) and the Maj and Tor Nessling foundation. We also acknowledge J. Keskinen and A. Arffman for fruitful discussions concerning impactor performance, H. Kuuluvainen for his help in bounce factor measurements, K. Rissa for SEM imaging, L. Hao, P. Tiitta, A. Kortelainen and P. Miettinen for aerosol mass spectrometer analyses and their help during experiments, J. Jokiniemi, U. Tapper and J. Lyyränen (VTT Technical Research Centre of Finland) for the development of SEM sample collection and image analysis methods, A. Diekmann for the differential scanning calorimeter measurements and T. Wagner for providing polystyrene samples.
Author information
Authors and Affiliations
Contributions
J.J., AV, J.K. and P.Y.-P. designed and conducted the plant chamber measurements and A.V., J.J., J.K., and M.K. the atmospheric measurements; A.V. developed the bounce factor concept; T.K. contributed the DSC data; J.K., A.V., J.J., J.L., P.Y.-P. and T.K. contributed to the interpretation of data; A.V. and T.K. wrote the manuscript; J.J., J.K., J.M.M., U.P., M.K., D.R.W. and A.L. discussed data and commented on the manuscript; A.L., M.K. and J.K.H. provided the working environment and financial support.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains a Supplementary Discussion, Supplementary Figures 1-8 with legends, Supplementary Methods, Supplementary Tables 1-2 and additional references. (PDF 1819 kb)
PowerPoint slides
Rights and permissions
About this article
Cite this article
Virtanen, A., Joutsensaari, J., Koop, T. et al. An amorphous solid state of biogenic secondary organic aerosol particles. Nature 467, 824–827 (2010). https://doi.org/10.1038/nature09455
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09455
This article is cited by
-
Analysis of the glass effect and Trommsdorff effect during bulk polymerization of methyl methacrylate, ethyl methacrylate, and butyl methacrylate
Polymer Journal (2023)
-
Chlorine activation and enhanced ozone depletion induced by wildfire aerosol
Nature (2023)
-
Polymerization-induced vitrification, apparent phase separation, and reaction acceleration during bulk polymerization
Polymer Journal (2023)
-
Formation of secondary organic aerosols from anthropogenic precursors in laboratory studies
npj Climate and Atmospheric Science (2022)
-
A biogenic secondary organic aerosol source of cirrus ice nucleating particles
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.