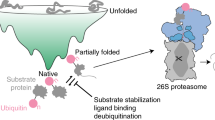
Abstract
Eukaryotes and archaea use a protease called the proteasome that has an integral role in maintaining cellular function through the selective degradation of proteins1,2,3,4. Proteolysis occurs in a barrel-shaped 20S core particle, which in Thermoplasma acidophilum is built from four stacked homoheptameric rings of subunits, α and β, arranged α7β7β7α7 (ref. 5). These rings form three interconnected cavities, including a pair of antechambers (formed by α7β7) through which substrates are passed before degradation and a catalytic chamber (β7β7) where the peptide-bond hydrolysis reaction occurs4,5. Although it is clear that substrates must be unfolded to enter through narrow, gated passageways (13 Å in diameter) located on the α-rings1,6,7, the structural and dynamical properties of substrates inside the proteasome antechamber remain unclear. Confinement in the antechamber might be expected to promote folding and thus impede proteolysis. Here we investigate the folding, stability and dynamics of three small protein substrates in the antechamber by methyl transverse-relaxation-optimized NMR spectroscopy8. We show that these substrates interact actively with the antechamber walls and have drastically altered kinetic and equilibrium properties that maintain them in unstructured states so as to be accessible for hydrolysis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Förster, A. & Hill, C. P. Proteasome degradation: enter the substrate. Trends Cell Biol. 13, 550–553 (2003)
Coux, O., Tanaka, K. & Goldberg, A. L. Structure and functions of the 20S and 26S proteasomes. Annu. Rev. Biochem. 65, 801–847 (1996)
Baumeister, W., Walz, J., Zühl, F. & Seemüller, E. The proteasome: paradigm of a self-compartmentalizing protease. Cell 92, 367–380 (1998)
Marques, A. J., Palanimurugan, R., Matias, A. C., Ramos, P. C. & Dohmen, R. J. Catalytic mechanism and assembly of the proteasome. Chem. Rev. 109, 1509–1536 (2009)
Löwe, J. et al. Crystal structure of the 20S proteasome from the archaeon T. acidophilum at 3.4 Å resolution. Science 268, 533–539 (1995)
Liu, C. W., Corboy, M. J., DeMartino, G. N. & Thomas, P. J. Endoproteolytic activity of the proteasome. Science 299, 408–411 (2003)
Cheng, Y. Toward an atomic model of the 26S proteasome. Curr. Opin. Struct. Biol. 19, 203–208 (2009)
Tugarinov, V., Hwang, P. M., Ollerenshaw, J. E. & Kay, L. E. Cross-correlated relaxation enhanced 1H-13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J. Am. Chem. Soc. 125, 10420–10428 (2003)
Sharon, M. et al. 20S proteasomes have the potential to keep substrates in store for continual degradation. J. Biol. Chem. 281, 9569–9575 (2006)
Hutschenreiter, S., Tinazli, A., Model, K. & Tampé, R. Two-substrate association with the 20S proteasome at single-molecule level. EMBO J. 23, 2488–2497 (2004)
Ellis, R. J. Protein folding: importance of the Anfinsen cage. Curr. Biol. 13, R881–R883 (2003)
Zhou, H. X. Protein folding in confined and crowded environments. Arch. Biochem. Biophys. 469, 76–82 (2008)
Ruschak, A. M. & Kay, L. E. Methyl groups as probes of supra-molecular structure, dynamics and function. J. Biomol. NMR 46, 75–87 (2010)
Mayor, U. et al. The complete folding pathway of a protein from nanoseconds to microseconds. Nature 421, 863–867 (2003)
Neudecker, P. et al. Identification of a collapsed intermediate with non-native long-range interactions on the folding pathway of a pair of Fyn SH3 domain mutants by NMR relaxation dispersion spectroscopy. J. Mol. Biol. 363, 958–976 (2006)
Jager, M. et al. Understanding the mechanism of beta-sheet folding from a chemical and biological perspective. Biopolymers 90, 751–758 (2008)
Zwickl, P., Kleinz, J. & Baumeister, W. Critical elements in proteasome assembly. Nature Struct. Biol. 1, 765–770 (1994)
Sprangers, R. & Kay, L. E. Quantitative dynamics and binding studies of the 20S proteasome by NMR. Nature 445, 618–622 (2007)
Wishart, D. S., Bigam, C. G., Holm, A., Hodges, R. S. & Sykes, B. D. 1H, 13C and 15N random coil NMR chemical shifts of the common amino acids. I. Investigations of nearest-neighbor effects. J. Biomol. NMR 5, 67–81 (1995)
Fitzkee, N. C. & Rose, G. D. Reassessing random-coil statistics in unfolded proteins. Proc. Natl Acad. Sci. USA 101, 12497–12502 (2004)
Jäger, M. et al. Structure-function-folding relationship in a WW domain. Proc. Natl Acad. Sci. USA 103, 10648–10653 (2006)
Kubelka, J., Hofrichter, J. & Eaton, W. A. The protein folding ‘speed limit’. Curr. Opin. Struct. Biol. 14, 76–88 (2004)
Jäger, M., Nguyen, H., Crane, J. C., Kelly, J. W. & Gruebele, M. The folding mechanism of a beta-sheet: the WW domain. J. Mol. Biol. 311, 373–393 (2001)
Tugarinov, V., Sprangers, R. & Kay, L. E. Probing side-chain dynamics in the proteasome by relaxation violated coherence transfer NMR spectroscopy. J. Am. Chem. Soc. 129, 1743–1750 (2007)
Choy, W. Y., Shortle, D. & Kay, L. E. Side chain dynamics in unfolded protein states: an NMR based 2H spin relaxation study of Δ131Δ. J. Am. Chem. Soc. 125, 1748–1758 (2003)
Battiste, J. L. & Wagner, G. Utilization of site-directed spin labeling and high-resolution heteronuclear nuclear magnetic resonance for global fold determination of large proteins with limited nuclear Overhauser effect data. Biochemistry 39, 5355–5365 (2000)
Clore, G. M., Tang, C. & Iwahara, J. Elucidating transient macromolecular interactions using paramagnetic relaxation enhancement. Curr. Opin. Struct. Biol. 17, 603–616 (2007)
Kisselev, A. F., Songyang, Z. & Goldberg, A. L. Why does threonine, and not serine, function as the active site nucleophile in proteasomes? J. Biol. Chem. 275, 14831–14837 (2000)
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995)
Fersht, A. Structure and Mechanism in Protein Science (Freeman, 1999)
Sprangers, R., Velyvis, A. & Kay, L. E. Solution NMR of supramolecular complexes: providing new insights into function. Nature Methods 4, 697–703 (2007)
Stollar, E. J. et al. Crystal structures of engrailed homeodomain mutants: implications for stability and dynamics. J. Biol. Chem. 278, 43699–43708 (2003)
Tugarinov, V. & Kay, L. E. Relaxation rates of degenerate 1H transitions in methyl groups of proteins as reporters of side-chain dynamics. J. Am. Chem. Soc. 128, 7299–7308 (2006)
Matsunaga, N. &. Nagashima, A. Transport properties of liquid and gaseous D2O over a wide range of temperature and pressure. J. Phys. Chem. 6, 1133–1166 (1977)
Acknowledgements
The authors would like to thank J. Forman-Kay for providing laboratory space and for discussions, R. Muhandiram for NMR support, H. Lin for help designing protein purification protocols, X. Li and A. Shimmer for assistance with the plate reader, and W. Baumeister for discussions. T.L.R. acknowledges The European Molecular Biology Organization (ALTF 827-2006) and The Canadian Institutes of Health Research (CIHR) for postdoctoral fellowships. L.E.K. holds a Canada Research Chair in Biochemistry. This work was supported by grants from the CIHR and the Natural Sciences and Engineering Research Council of Canada.
Author information
Authors and Affiliations
Contributions
A.M.R. made samples. T.L.R. helped make plasmids for protein expression and trained A.M.R. to express and purify proteins. A.M.R. and L.E.K. designed experiments, recorded and analysed NMR data, and wrote the manuscript. S.B. and S.W. were involved in preliminary experimental design, and S.W. and T.L.R. commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-12 with legends, Supplementary Table 1 and additional references. (PDF 4049 kb)
Rights and permissions
About this article
Cite this article
Ruschak, A., Religa, T., Breuer, S. et al. The proteasome antechamber maintains substrates in an unfolded state. Nature 467, 868–871 (2010). https://doi.org/10.1038/nature09444
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature09444
This article is cited by
-
Protein degradation by human 20S proteasomes elucidates the interplay between peptide hydrolysis and splicing
Nature Communications (2024)
-
Spectral editing of intra- and inter-chain methyl–methyl NOEs in protein complexes
Journal of Biomolecular NMR (2020)
-
Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice
Nature Genetics (2015)
-
Scrambling free combinatorial labeling of alanine-β, isoleucine-δ1, leucine-proS and valine-proS methyl groups for the detection of long range NOEs
Journal of Biomolecular NMR (2015)
-
Assignment of methyl NMR resonances of a 52 kDa protein with residue-specific 4D correlation maps
Journal of Biomolecular NMR (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.