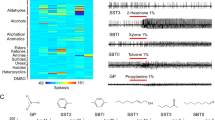
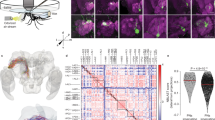
Abstract
The mosquito Anopheles gambiae is the major vector of malaria in sub-Saharan Africa. It locates its human hosts primarily through olfaction, but little is known about the molecular basis of this process. Here we functionally characterize the Anopheles gambiae odorant receptor (AgOr) repertoire. We identify receptors that respond strongly to components of human odour and that may act in the process of human recognition. Some of these receptors are narrowly tuned, and some salient odorants elicit strong responses from only one or a few receptors, suggesting a central role for specific transmission channels in human host-seeking behaviour. This analysis of the Anopheles gambiae receptors permits a comparison with the corresponding Drosophila melanogaster odorant receptor repertoire. We find that odorants are differentially encoded by the two species in ways consistent with their ecological needs. Our analysis of the Anopheles gambiae repertoire identifies receptors that may be useful targets for controlling the transmission of malaria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
World Health Organization. World Malaria Report 〈http://www.who.int/malaria/publications/atoz/9789241563697/en/index.html〉 (2008)
Zwiebel, L. J. & Takken, W. Olfactory regulation of mosquito-host interactions. Insect Biochem. Mol. Biol. 34, 645–652 (2004)
Takken, W. The role of olfaction in host-seeking of mosquitos: a review. Insect Sci. Applic. 12, 287–295 (1991)
Takken, W. & Knols, B. G. J. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu. Rev. Entomol. 44, 131–157 (1999)
Su, C. Y., Menuz, K. & Carlson, J. R. Olfactory perception: receptors, cells, and circuits. Cell 139, 45–59 (2009)
Hill, C. A. et al. G protein coupled receptors in Anopheles gambiae . Science 298, 176–178 (2002)
Fox, A. N., Pitts, R. J., Robertson, H. M., Carlson, J. R. & Zwiebel, L. J. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc. Natl Acad. Sci. USA 98, 14693–14697 (2001)
Hallem, E. A., Fox, A. N., Zwiebel, L. J. & Carlson, J. R. Olfaction: mosquito receptor for human-sweat odorant. Nature 427, 212–213 (2004)
Dobritsa, A. A., van der Goes van Naters, W., Warr, C. G., Steinbrecht, R. A. & Carlson, J. R. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron 37, 827–841 (2003)
Kreher, S. A., Mathew, D., Kim, J. & Carlson, J. R. Translation of sensory input into behavioral output via an olfactory system. Neuron 59, 110–124 (2008)
Hallem, E. A., Ho, M. G. & Carlson, J. R. The molecular basis of odor coding in the Drosophila antenna. Cell 117, 965–979 (2004)
Hallem, E. A. & Carlson, J. R. Coding of odors by a receptor repertoire. Cell 125, 143–160 (2006)
Gaunt, M. W. & Miles, M. A. An insect molecular clock dates the origin of the insects and accords with palaeontological and biogeographic landmarks. Mol. Biol. Evol. 19, 748–761 (2002)
Lu, T. et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae . Curr. Biol. 17, 1533–1544 (2007)
Malnic, B., Hirono, J., Sato, T. & Buck, L. B. Combinatorial receptor codes for odors. Cell 96, 713–723 (1999)
Saito, H., Chi, Q., Zhuang, H., Matsunami, H. & Mainland, J. D. Odor coding by a mammalian receptor repertoire. Sci. Signal. 2, ra9 (2009)
Wilson, R. I. & Mainen, Z. F. Early events in olfactory processing. Annu. Rev. Neurosci. 29, 163–201 (2006)
Meijerink, J. et al. Identification of olfactory stimulants for Anopheles gambiae from human sweat samples. J. Chem. Ecol. 26, 1367–1382 (2000)
Verhulst, N. O. et al. Cultured skin microbiota attracts malaria mosquitoes. Malar. J. 8, 302 (2009)
Sun, H. et al. Alcohol, volatile fatty acid, phenol, and methane emissions from dairy cows and fresh manure. J. Environ. Qual. 37, 615–622 (2008)
Gutiérrez-García, A. G., Contreras, C. M., Mendoza-Lopez, M. R., Garcia-Barradas, O. & Cruz-Sanchez, J. S. Urine from stressed rats increases immobility in receptor rats forced to swim: role of 2-heptanone. Physiol. Behav. 91, 166–172 (2007)
TNO. Volatile Compounds in Food: Qualitative and Quantitative Data 〈http://www.vcf-online.nl〉 (2004)
Morton, I. D. & MacLeod, A. J. The Flavour Of Fruits (Elsevier Science, 1990)
Millar, J. G., Chaney, J. D. & Mulla, M. S. Identification of oviposition attractants for culex-quinquefasciatus from fermented bermuda grass infusions. J. Am. Mosq. Control Assoc. 8, 11–17 (1992)
Syed, Z. & Leal, W. S. Acute olfactory response of Culex mosquitoes to a human- and bird-derived attractant. Proc. Natl Acad. Sci. USA 106, 18803–18808 (2009)
Blackwell, A. & Johnson, S. N. Electrophysiological investigation of larval water and potential oviposition chemo-attractants for Anopheles gambiae s.s. Ann. Trop. Med. Parasitol. 94, 389–398 (2000)
Lindh, J. M., Kannaste, A., Knols, B. G. J., Faye, I. & Borg-Karlson, A. K. Oviposition responses of Anopheles Gambiae s.s. (Diptera: Culicidae) and identification of volatiles from bacteria-containing solutions. J. Med. Entomol. 45, 1039–1049 (2008)
Kostelc, J. G., Preti, G., Zelson, P. R., Stoller, N. H. & Tonzetich, J. Salivary volatiles as indicators of periodontitis. J. Periodontal Res. 15, 185–192 (1980)
Moore, S. J. & Debboun, M. in Insect Repellents: Principles, Methods, and Uses (eds Debboun, M., Frances, S. P. & Strickman, D.) 3–30 (CRC, 2007)
Omolo, M. O., Okinyo, D., Ndiege, I. O., Lwande, W. & Hassanali, A. Repellency of essential oils of some Kenyan plants against Anopheles gambiae . Phytochemistry 65, 2797–2802 (2004)
Tangerman, A., Meuwesearends, M. T. & Vantongeren, J. H. M. A new sensitive assay for measuring volatile sulfur-compounds in human breath by tenax trapping and gas-chromatography and its application in liver-cirrhosis. clin. Chim. Acta 130, 103–110 (1983)
Allan, S. A., Bernier, U. R. & Kline, D. L. Evaluation of oviposition substrates and organic infusions on collection of Culex in Florida. J. Am. Mosq. Control Assoc. 21, 268–273 (2005)
Haddad, R. et al. A metric for odorant comparison. Nature Methods 5, 425–429 (2008)
Logan, J. G. et al. Identification of human-derived volatile chemicals that interfere with attraction of Aedes aegypti mosquitoes. J. Chem. Ecol. 34, 308–322 (2008)
Curran, A. M., Ramirez, C. F., Schoon, A. A. & Furton, K. G. The frequency of occurrence and discriminatory power of compounds found in human scent across a population determined by SPME-GEMS. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 846, 86–97 (2007)
Curran, A. M., Rabin, S. I., Prada, P. A. & Furton, K. G. Comparison of the volatile organic compounds present in human odor using SPME-GC/MS. J. Chem. Ecol. 31, 1607–1619 (2005)
Cork, A. & Park, K. C. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med. Vet. Entomol. 10, 269–276 (1996)
Bernier, U. R., Kline, D. L., Schreck, C. E., Yost, R. A. & Barnard, D. R. Chemical analysis of human skin emanations: comparison of volatiles from humans that differ in attraction of Aedes aegypti (Diptera: Culicidae). J. Am. Mosq. Control Assoc. 18, 186–195 (2002)
Bernier, U. R., Kline, D. L., Barnard, D. R., Schreck, C. E. & Yost, R. A. Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal. Chem. 72, 747–756 (2000)
Gallagher, M. et al. Analyses of volatile organic compounds from human skin. Br. J. Dermatol. 159, 780–791 (2008)
Kreher, S. A., Kwon, J. Y. & Carlson, J. R. The molecular basis of odor coding in the Drosophila larva. Neuron 46, 445–456 (2005)
Benton, R., Vannice, K. S., Gomez-Diaz, C. & Vosshall, L. B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila . Cell 136, 149–162 (2009)
Olsen, S. R. & Wilson, R. I. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 452, 956–960 (2008)
Schlief, M. L. & Wilson, R. I. Olfactory processing and behavior downstream from highly selective receptor neurons. Nature Neurosci. 10, 623–630 (2007)
Yao, C. A., Ignell, R. & Carlson, J. R. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J. Neurosci. 25, 8359–8367 (2005)
Larsson, M. C. et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714 (2004)
Xia, Y. et al. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc. Natl Acad. Sci. USA 105, 6433–6438 (2008)
Acknowledgements
We thank W. van der Goes van Naters and C. Yao for help with electrophysiology, E. Hallem, S. Kreher, J. Salzman and T. Emonet for assistance with data analyses, and T.-W. Koh for comments on the manuscript. We thank P. Graham, Z. Berman, A. Rabin, M. Dillon and E. Kelley-Swift for technical assistance. We thank Y.-T. Qiu for assistance in generating Fig. 1b and Supplementary Fig. 1. This work was funded in part by grants from the Foundation for the National Institutes of Health (NIH) through the Grand Challenges in Global Health Initiative to L.J.Z., and from the NIH to L.J.Z. and J.R.C. A.F.C. is supported by an NIH Medical Scientist Training Program grant (2T32GM07205).
Author Contributions Electrophysiology and computational analysis were performed by A.F.C. Molecular cloning was performed by A.F.C., G.W. and C.-Y.S. A.F.C. and J.R.C. wrote the manuscript. All authors contributed to the design and interpretation of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-8 with Legends, Supplementary Tables 1 & 3 Supplementary References and a Legend and References for Supplementary Table 2. (PDF 829 kb)
Supplementary Table
This file contains Supplementary Table 2 - see Supplementary Information file for Legend. (XLS 383 kb)
Rights and permissions
About this article
Cite this article
Carey, A., Wang, G., Su, CY. et al. Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464, 66–71 (2010). https://doi.org/10.1038/nature08834
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08834
This article is cited by
-
An odorant receptor mediates the avoidance of Plutella xylostella against parasitoid
BMC Biology (2024)
-
Identification of an adult attractant for Anomala corpulenta by the reverse chemical ecology approach
Journal of Pest Science (2024)
-
AsOBP1 is required for host seeking in the malaria vector mosquito, Anopheles sinensis
Journal of Pest Science (2024)
-
High-throughput ligand profile characterization in novel cell lines expressing seven heterologous insect olfactory receptors for the detection of volatile plant biomarkers
Scientific Reports (2023)
-
Combinatorial encoding of odors in the mosquito antennal lobe
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.