Abstract
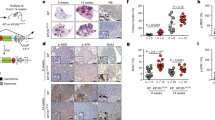
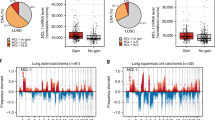
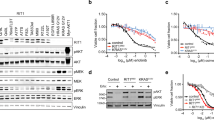
Inhibition of an initiating oncogene often leads to extensive tumour cell death, a phenomenon known as oncogene addiction1. This has led to the search for compounds that specifically target and inhibit oncogenes as anticancer agents. However, there has been no systematic exploration of whether chromosomal instability generated as a result of deregulation of the mitotic checkpoint pathway2,3, a frequent characteristic of solid tumours, has any effect on oncogene addiction. Here we show that induction of chromosome instability by overexpression of the mitotic checkpoint gene Mad2 in mice does not affect the regression of Kras-driven lung tumours when Kras is inhibited. However, tumours that experience transient Mad2 overexpression and consequent chromosome instability recur at markedly elevated rates. The recurrent tumours are highly aneuploid and have varied activation of pro-proliferative pathways. Thus, early chromosomal instability may be responsible for tumour relapse after seemingly effective anticancer treatments.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Weinstein, I. B. & Joe, A. K. Mechanisms of disease: oncogene addiction—a rationale for molecular targeting in cancer therapy. Naure. Clin. Pract. Oncol. 3, 448–457 (2006)
Hernando, E. et al. Rb inactivation promotes genomic instability by uncoupling cell cycle progression from mitotic control. Nature 430, 797–802 (2004)
Sotillo, R. et al. Mad2 overexpression promotes aneuploidy and tumorigenesis in mice. Cancer Cell 11, 9–23 (2007)
Schvartzman, J. M., Sotillo, R. & Benezra, R. Mitotic chromosomal instability and cancer: mouse modelling of the human disease. Nature Rev. Cancer 10, 102–115 (2010)
Ricke, R. M., van Ree, J. H. & van Deursen, J. M. Whole chromosome instability and cancer: a complex relationship. Trends Genet. 24, 457–466 (2008)
Chang, S., Khoo, C. & DePinho, R. A. Modeling chromosomal instability and epithelial carcinogenesis in the telomerase-deficient mouse. Semin. Cancer Biol. 11, 227–239 (2001)
Tichelaar, J. W., Lu, W. & Whitsett, J. A. Conditional expression of fibroblast growth factor-7 in the developing and mature lung. J. Biol. Chem. 275, 11858–11864 (2000)
Fisher, G. H. et al. Induction and apoptotic regression of lung adenocarcinomas by regulation of a K-Ras transgene in the presence and absence of tumor suppressor genes. Genes Dev. 15, 3249–3262 (2001)
Chin, L. et al. Essential role for oncogenic Ras in tumour maintenance. Nature 400, 468–472 (1999)
Felsher, D. W. & Bishop, J. M. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol. Cell 4, 199–207 (1999)
Giuriato, S. & Felsher, D. W. How cancers escape their oncogene habit. Cell Cycle 2, 329–332 (2003)
Boxer, R. B., Jang, J. W., Sintasath, L. & Chodosh, L. A. Lack of sustained regression of c-MYC-induced mammary adenocarcinomas following brief or prolonged MYC inactivation. Cancer Cell 6, 577–586 (2004)
Gorre, M. E. et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science (New York, N. Y.) 293, 876–880 (2001)
Kobayashi, S. et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 352, 786–792 (2005)
Pao, W. et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2, e73 (2005)
Politi, K. et al. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes Dev. 20, 1496–1510 (2006)
Baker, D. J., Chen, J. & van Deursen, J. M. The mitotic checkpoint in cancer and aging: what have mice taught us? Curr. Opin. Cell Biol. 17, 583–589 (2005)
Pérez de Castro, I., de Cárcer, G. & Malumbres, M. A census of mitotic cancer genes: new insights into tumor cell biology and cancer therapy. Carcinogenesis 28, 899–912 (2007)
Dobles, M., Liberal, V., Scott, M. L., Benezra, R. & Sorger, P. K. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell 101, 635–645 (2000)
Li, M., York, J. P. & Zhang, P. Loss of Cdc20 causes a securin-dependent metaphase arrest in two-cell mouse embryos. Mol. Cell. Biol. 27, 3481–3488 (2007)
Kwon, M. et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 22, 2189–2203 (2008)
Weaver, B. A., Silk, A. D., Montagna, C., Verdier-Pinard, P. & Cleveland, D. W. Aneuploidy acts both oncogenically and as a tumor suppressor. Cancer Cell 11, 25–36 (2007)
Rao, C. V. et al. Colonic tumorigenesis in BubR1+/-ApcMin/+ compound mutant mice is linked to premature separation of sister chromatids and enhanced genomic instability. Proc. Natl Acad. Sci. USA 102, 4365–4370 (2005)
Jeganathan, K., Malureanu, L., Baker, D. J., Abraham, S. C. & van Deursen, J. M. Bub1 mediates cell death in response to chromosome missegregation and acts to suppress spontaneous tumorigenesis. J. Cell Biol. 179, 255–267 (2007)
Druker, B. J. et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N. Engl. J. Med. 344, 1031–1037 (2001)
Cortes, J. & O’Dwyer, M. E. Clonal evolution in chronic myelogenous leukemia. Hematol. Oncol. Clin. North Am. 18, 671–684 (2004)
Hochhaus, A. et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia 16, 2190–2196 (2002)
O’Dwyer, M. E. et al. The impact of clonal evolution on response to imatinib mesylate (STI571) in accelerated phase CML. Blood 100, 1628–1633 (2002)
Acknowledgements
We thank H. Varmus and W. Pao for the CCSP–rtTA and TetO–Kras mice; K. Politi, K. Podsypanina and M. Jechlinger for reagents, discussions and critical reading of the manuscript; C. Le and M. Lupu for MR imaging; M. Leversha, C. Kalyani and J. McGuire for FISH staining; K. M. D. La Perle, M. Jiao and M. Squatrito for immunohistochemistry and pathological analysis; A. Viale for CGH and Affymetrix; and Y. Chin, S. Curelariu and C. Coker for excellent technical assistance. Support was provided to R.S. by the Charles H. Revson Foundation, to J.M.S. by a Breast Cancer Research Program (BCRP) Predoctoral Traineeship Award from the US Department of Defense (Congressionally Directed Medical Research Programs) and to R.B. by the National Institutes of Health.
Author Contributions R.S., J.M.S. and R.B. designed the study. R.S. performed experiments. R.S., J.M.S., N.S. and R.B. analysed the data. R.S., J.M.S. and R.B. wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-9 with Legends and Supplementary Tables 1-3. (PDF 6331 kb)
Rights and permissions
About this article
Cite this article
Sotillo, R., Schvartzman, JM., Socci, N. et al. Mad2-induced chromosome instability leads to lung tumour relapse after oncogene withdrawal. Nature 464, 436–440 (2010). https://doi.org/10.1038/nature08803
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08803
This article is cited by
-
Unraveling the Drivers of Tumorigenesis in the Context of Evolution: Theoretical Models and Bioinformatics Tools
Journal of Molecular Evolution (2023)
-
The positive feedback loop of MAD2L1/TYK2/STAT3 induces progression in B-cell acute lymphoblastic leukaemia
Journal of Cancer Research and Clinical Oncology (2023)
-
Disentangling the roles of aneuploidy, chromosomal instability and tumour heterogeneity in developing resistance to cancer therapies
Chromosome Research (2023)
-
Predictive validity in drug discovery: what it is, why it matters and how to improve it
Nature Reviews Drug Discovery (2022)
-
Effects of aneuploidy on cell behaviour and function
Nature Reviews Molecular Cell Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.