Abstract
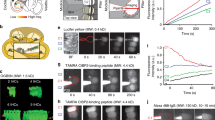
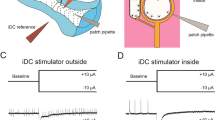
The mammalian cochlea is innervated by two classes of sensory neurons. Type I neurons make up 90–95% of the cochlear nerve and contact single inner hair cells to provide acoustic analysis as we know it. In contrast, the far less numerous type II neurons arborize extensively among outer hair cells (OHCs)1,2 and supporting cells3,4. Their scarcity and smaller calibre axons have made them the subject of much speculation, but little experimental progress for the past 50 years. Here we record from type II fibres near their terminal arbors under OHCs to show that they receive excitatory glutamatergic synaptic input. The type II peripheral arbor conducts action potentials, but the small and infrequent glutamatergic excitation indicates a requirement for strong acoustic stimulation. Furthermore, we show that type II neurons are excited by ATP. Exogenous ATP depolarized type II neurons, both directly and by evoking glutamatergic synaptic input5. These results prove that type II neurons function as cochlear afferents, and can be modulated by ATP. The lesser magnitude of synaptic drive dictates a fundamentally different role in auditory signalling from that of type I afferents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Perkins, R. E. & Morest, D. K. A study of cochlear innervation patterns in cats and rats with the Golgi method and Nomarkski Optics. J. Comp. Neurol. 163, 129–158 (1975)
Berglund, A. M. & Ryugo, D. K. Hair cell innervation by spiral ganglion neurons in the mouse. J. Comp. Neurol. 255, 560–570 (1987)
Fechner, F. P., Nadol, J. J., Burgess, B. J. & Brown, M. C. Innervation of supporting cells in the apical turns of the guinea pig cochlea is from type II afferent fibers. J. Comp. Neurol. 429, 289–298 (2001)
Brown, M. C. Morphology of labeled afferent fibers in the guinea pig cochlea. J. Comp. Neurol. 260, 591–604 (1987)
Nakagawa, T., Akaike, N., Kimitsuki, T., Komune, S. & Arima, T. ATP-induced current in isolated outer hair cells of guinea pig cochlea. J. Neurophysiol. 63, 1068–1074 (1990)
Lorente de No, R. The sensory endings in the cochlea. Laryngoscope 47, 373–377 (1937)
Michna, M. et al. Cav1.3 (α1D) Ca2+ currents in neonatal outer hair cells of mice. J. Physiol. (Lond.) 553, 747–758 (2003)
Glowatzki, E. & Fuchs, P. A. Transmitter release at the hair cell ribbon synapse. Nature Neurosci. 5, 147–154 (2002)
Järlebark, L. E., Housley, G. D. & Thorne, P. R. Immunohistochemical localization of adenosine 5′-triphosphate-gated ion channel P2X2 receptor subunits in adult and developing rat cochlea. J. Comp. Neurol. 421, 289–301 (2000)
Robertson, D. Horseradish peroxidase injection of physiologically characterized afferent and efferent neurones in the guinea pig spiral ganglion. Hear. Res. 15, 113–121 (1984)
Jagger, D. J. & Housley, G. D. Membrane properties of type II spiral ganglion neurones identified in a neonatal rat cochlear slice. J. Physiol. (Lond.) 552, 525–533 (2003)
Reid, M. A., Flores-Otero, J. & Davis, R. L. Firing patterns of type II spiral ganglion neurons in vitro . J. Neurosci. 24, 733–742 (2004)
Goutman, J. D. & Glowatzki, E. Time course and calcium dependence of transmitter release at a single ribbon synapse. Proc. Natl Acad. Sci. USA 104, 16341–16346 (2007)
Thiers, F. A., Nadol, J. B. & Liberman, M. C. Reciprocal synapses between outer hair cells and their afferent terminals: evidence for a local neural network in the mammalian cochlea. J. Assoc. Res. Otolaryngol. 9, 477–489 (2008)
Matsubara, A., Laake, J. H., Davanger, S., Usami, S. & Ottersen, O. P. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J. Neurosci. 16, 4457–4467 (1996)
Knirsch, M. et al. Persistence of Cav1.3 Ca2+ channels in mature outer hair cells supports outer hair cell afferent signaling. J. Neurosci. 27, 6442–6451 (2007)
Beurg, M. et al. Calcium- and otoferlin-dependent exocytosis by immature outer hair cells. J. Neurosci. 28, 1798–1803 (2008)
Dunn, R. A. & Morest, D. K. Receptor synapses without synaptic ribbons in the cochlea of the cat. Proc. Natl Acad. Sci. USA 72, 3599–3603 (1975)
Hashimoto, S. & Kimura, R. S. Computer-aided three-dimensional reconstruction and morphometry of the outer hair cells of the guinea pig cochlea. Acta Otolaryngol. (Stockh.) 105, 64–74 (1988)
Simmons, D. D. & Liberman, M. C. Afferent innervation of outer hair cells in adult cats: II. Electron microscopic analysis of fibers labeled with horseradish peroxidase. J. Comp. Neurol. 270, 145–154 (1988)
Burgess, B. J., Adams, J. C. & Nadol, J. B. Morphologic evidence for innervation of Deiters’ and Hensen’s cells in the guinea pig. Hear. Res. 108, 74–82 (1997)
Neef, A. et al. Probing the mechanism of exocytosis at the hair cell ribbon synapse. J. Neurosci. 27, 12933–12944 (2007)
Berglund, A. M. & Brown, M. C. Central trajectories of type II spiral ganglion cells from various cochlear regions in mice. Hear. Res. 75, 121–130 (1994)
Huang, L. C., Greenwood, D., Thorne, P. R. & Housley, G. D. Developmental regulation of neuron-specific P2X3 receptor expression in the rat cochlea. J. Comp. Neurol. 484, 133–143 (2005)
Tritsch, N. X., Yi, E., Gale, J. E., Glowatzki, E. & Bergles, D. E. The origin of spontaneous activity in the developing auditory system. Nature 450, 50–55 (2007)
Brown, M. C., Liberman, M. C., Benson, T. E. & Ryugo, D. K. Brainstem branches from olivocochlear axons in cats and rodents. J. Comp. Neurol. 278, 591–603 (1988)
Gale, J. E., Piazza, V., Ciubotaru, C. D. & Mammano, F. A mechanism for sensing noise damage in the inner ear. Curr. Biol. 14, 526–529 (2004)
Muñoz, D. J., Kendrick, I. S., Rassam, M. & Thorne, P. R. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngol. (Stockh.) 121, 10–15 (2001)
Burnstock, G. Purinergic receptors and pain. Curr. Pharm. Des. 15, 1717–1735 (2009)
Wang, J. C. et al. Noise induces up-regulation of P2X2 receptor subunit of ATP-gated ion channels in the rat cochlea. Neuroreport 14, 817–823 (2003)
Acknowledgements
Supported by NIDCD grants R01 DC000276 and R01 DC006476, T32 DC000023 and a grant from the Blaustein Pain Foundation of Johns Hopkins.
Author Contributions C.W. performed and analysed all experiments with further analysis from P.F. and E.G. C.W., E.G. and P.F. conceived the project, designed and discussed the experiments, and wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Tables 1-3 and Supplementary Figure 1 with Legends and Supplementary References to Table 1. (PDF 165 kb)
Rights and permissions
About this article
Cite this article
Weisz, C., Glowatzki, E. & Fuchs, P. The postsynaptic function of type II cochlear afferents. Nature 461, 1126–1129 (2009). https://doi.org/10.1038/nature08487
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature08487
This article is cited by
-
Acoustic Trauma Increases Ribbon Number and Size in Outer Hair Cells of the Mouse Cochlea
Journal of the Association for Research in Otolaryngology (2021)
-
Disruption of Atg7-dependent autophagy causes electromotility disturbances, outer hair cell loss, and deafness in mice
Cell Death & Disease (2020)
-
Purinergic Modulation of Activity in the Developing Auditory Pathway
Neuroscience Bulletin (2020)
-
Characterization of transgenic mouse lines for labeling type I and type II afferent neurons in the cochlea
Scientific Reports (2019)
-
Neuronal heterogeneity and stereotyped connectivity in the auditory afferent system
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.