Abstract
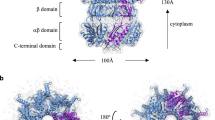
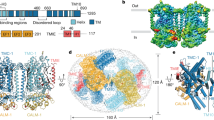
The ability of cells to sense and respond to mechanical force underlies diverse processes such as touch and hearing in animals, gravitropism in plants, and bacterial osmoregulation1,2. In bacteria, mechanosensation is mediated by the mechanosensitive channels of large (MscL), small (MscS), potassium-dependent (MscK) and mini (MscM) conductances. These channels act as ‘emergency relief valves’ protecting bacteria from lysis upon acute osmotic down-shock3. Among them, MscL has been intensively studied since the original identification and characterization 15 years ago4. MscL is reversibly and directly gated by changes in membrane tension. In the open state, MscL forms a non-selective 3 nS conductance channel which gates at tensions close to the lytic limit of the bacterial membrane. An earlier crystal structure at 3.5 Å resolution of a pentameric MscL from Mycobacterium tuberculosis represents a closed-state or non-conducting conformation5,6. MscL has a complex gating behaviour; it exhibits several intermediates between the closed and open states, including one putative non-conductive expanded state and at least three sub-conducting states7. Although our understanding of the closed5,6 and open8,9,10 states of MscL has been increasing, little is known about the structures of the intermediate states despite their importance in elucidating the complete gating process of MscL. Here we present the crystal structure of a carboxy-terminal truncation mutant (Δ95–120) of MscL from Staphylococcus aureus (SaMscL(CΔ26)) at 3.8 Å resolution. Notably, SaMscL(CΔ26) forms a tetrameric channel with both transmembrane helices tilted away from the membrane normal at angles close to that inferred for the open state9, probably corresponding to a non-conductive but partially expanded intermediate state.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kung, C. A possible unifying principle for mechanosensation. Nature 436, 647–654 (2005)
Sukharev, S. & Corey, D. P. Mechanosensitive channels: multiplicity of families and gating paradigms. Sci. STKE 2004, 1–24 (2004)
Booth, I. R., Edwards, M. D., Black, S., Schumann, U. & Miller, S. Mechanosensitive channels in bacteria: signs of closure? Nature Rev. Microbiol. 5, 431–440 (2007)
Sukharev, S. I., Blount, P., Martinac, B., Blattner, F. R. & Kung, C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 368, 265–268 (1994)
Chang, G., Spencer, R. H., Lee, A. T., Barclay, M. T. & Rees, D. C. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science 282, 2220–2226 (1998)
Steinbacher, S., Bass, R., Strop, P. & Rees, D. C. in Current Topics in Membranes. Mechanosensitive Ion Channels, Part A (ed. Hamill, O. P.) 1–24 (Academic, 2007)
Sukharev, S. I., Sigurdson, W. J., Kung, C. & Sachs, F. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J. Gen. Physiol. 113, 525–540 (1999)
Sukharev, S., Betanzos, M., Chiang, C.-S. & Guy, H. R. The gating mechanism of the large mechanosensitive channel MscL. Nature 409, 720–724 (2001)
Perozo, E., Cortes, D. M., Sompornpisut, P., Kloda, A. & Martinac, B. Open channel structure of MscL and the gating mechanism of mechanosensitive channels. Nature 418, 942–948 (2002)
Anishkin, A. & Sukharev, S. State-stabilizing interactions in the bacterial mechanosensitive channel gating and adaptation. J. Biol. Chem. 284, 19153–19157 (2009)
Moe, P. C., Blount, P. & Kung, C. Functional and structural conservation in the mechanosensitive channel MscL implicates elements crucial for mechanosensation. Mol. Microbiol. 28, 583–592 (1998)
Levina, N. et al. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 18, 1730–1737 (1999)
Blount, P., Sukharev, S. I., Schroeder, M. J., Nagle, S. K. & Kung, C. Single residue substitutions that change the gating properties of a mechanosensitive channel in Escherichia coli. Proc. Natl Acad. Sci. USA 93, 11652–11657 (1996)
Häse, C. C., Le Dain, A. C. & Martinac, B. Molecular dissection of the large mechanosensitive ion channel (MscL) of E. coli: mutants with altered channel gating and pressure sensitivity. J. Membr. Biol. 157, 17–25 (1997)
Niegowski, D. & Eshaghi, S. The CorA family: structure and function revisited. Cell. Mol. Life Sci. 64, 2564–2574 (2007)
Cogdell, R. J. et al. The structure and function of the LH2 (B800–850) complex from the purple photosynthetic bacterium Rhodopseudomonas acidophila strain 10050. Prog. Biophys. Mol. Biol. 68, 1–27 (1997)
Stock, D., Leslie, A. G. & Walker, J. E. Molecular architecture of the rotary motor in ATP synthase. Science 286, 1700–1705 (1999)
Sukharev, S., Durell, S. R. & Guy, H. R. Structural models of the MscL gating mechanism. Biophys. J. 81, 917–936 (2001)
Strop, P., Bass, R. & Rees, D. C. Prokaryotic mechanosensitive channels. Adv. Protein Chem. 63, 177–209 (2003)
Beckstein, O. & Sansom, M. S. P. The influence of geometry, surface character, and flexibility on the permeation of ions and water through biological pores. Phys. Biol. 1, 42–52 (2004)
Cruickshank, C. C., Minchin, R. F., Le Dain, A. C. & Martinac, B. Estimation of the pore size of the large-conductance mechanosensitive ion channel of Escherichia coli. Biophys. J. 73, 1925–1931 (1997)
Ajouz, B., Berrier, C., Garrigues, A., Besnard, M. & Ghazi, A. Release of thioredoxin via the mechanosensitive channel MscL during osmotic downshock of Escherichia coli cells. J. Biol. Chem. 273, 26670–26674 (1998)
van den Bogaart, G., Krasnikov, V. & Poolman, B. Dual-color fluorescence-burst analysis to probe protein efflux through the mechanosensitive channel MscL. Biophys. J. 92, 1233–1240 (2007)
Yoshimura, K., Usukura, J. & Sokabe, M. Gating-associated conformational changes in the mechanosensitive channel MscL. Proc. Natl Acad. Sci. USA 105, 4033–4038 (2008)
Strop, P. & Brunger, A. T. Refractive index-based determination of detergent concentration and its application to the study of membrane proteins. Protein Sci. 14, 2207–2211 (2005)
Spencer, R. H. & Rees, D. C. The α-helix and the organization and gating of channels. Annu. Rev. Biophys. Biomol. Struct. 31, 207–233 (2002)
Wang, W. et al. The structure of an open form of an E. coli mechanosensitive channel at 3.45 Å resolution. Science 321, 1179–1183 (2008)
Maurer, J. A., Elmore, D. E., Lester, H. A. & Dougherty, D. A. Comparing and contrasting Escherichia coli and Mycobacterium tuberculosis mechanosensitive channels (MscL). J. Biol. Chem. 275, 22238–22244 (2000)
Tsai, I. J. et al. The role of the periplasmic loop residue glutamine 65 for MscL mechanosensitivity. Eur. Biophys. J. 34, 403–412 (2005)
Ajouz, B., Berrier, C., Besnard, M., Martinac, B. & Ghazi, A. Contributions of the different extramembranous domains of the mechanosensitive ion channel MscL to its response to membrane tension. J. Biol. Chem. 275, 1015–1022 (2000)
Lusty, C. A gentle vapor-diffusion technique for cross-linking of protein crystals for cryocrystallography. J. Appl. Cryst. 32, 106–112 (1999)
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008)
Read, R. Improved Fourier coefficients for maps using phases from partial structures with errors. Acta Crystallogr. A 42, 140–149 (1986)
Collaborative Computational Project, 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Cowtan, K. DM: an automated procedure for phase improvement by density modification. Joint CCP4 ESF-EACBM Newslett. Protein Crystallogr. 31, 34–38 (1994)
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47, 110–119 (1991)
Brunger, A. T. Version 1.2 of the crystallography and NMR system. Nature Protocols 2, 2728–2733 (2007)
Laskowski, R. A., MacArthur, M. W., Moss, D. S. & Thornton, J. M. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 26, 283–291 (1993)
Larkin, M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007)
Gouet, P., Courcelle, E., Stuart, D. I. & Metoz, F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15, 305–308 (1999)
DeLano, W. L. The PyMOL User's Manual (Delano Scientific, 2002)
Smart, O. S., Neduvelil, J. G., Wang, X. N., Wallace, B. A. & Sansom, M. S. P. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. Model. 14, 354–360 (1996)
Hutchinson, E. G. & Thornton, J. M. PROMOTIF–a program to identify and analyze structural motifs in proteins. Protein Sci. 5, 212–220 (1996)
Iscla, I., Wray, R. & Blount, P. On the structure of the N-terminal domain of the MscL channel: helical bundle or membrane interface. Biophys. J. 95, 2283–2291 (2008)
Häse, C. C., Le Dain, A. C. & Martinac, B. Purification and functional reconstitution of the recombinant large mechanosensitive ion channel (MscL) of Escherichia coli. J. Biol. Chem. 270, 18329–18334 (1995)
Sukharev, S. I., Martinac, B., Arshavsky, V. Y. & Kung, C. Two types of mechanosensitive channels in the Escherichia coli cell envelope: solubilization and functional reconstitution. Biophys. J. 65, 177–183 (1993)
Acknowledgements
We thank A. Shih for early cloning work, T. Walton for biochemical analysis, A. Lee for initial efforts on the MscL project, J. Choe for expression and purification protocols, O. Lewinson for manuscript reading, Y. Poon and J. Lai for treating MJF465 with λDE3 and the osmotic down-shock assay protocol, J. Kaiser for suggestions on structure refinement, R. Phillips, E. Haswell and P. Pal for discussions, P. Blount for the MJF465 strain, and the staff at SSRL, the Advanced Light Source (ALS) and the Advanced Photon Source (APS) for technical support with crystal screening and data collection. We would like to acknowledge the Gordon and Betty Moore Foundation for support of the Molecular Observatory at Caltech. Operations at SSRL, ALS and APS are supported by the US Department of Energy and NIH. This work was supported in part by grants from the Howard Hughes Medical Institute and the National Institutes of Health (GM084211). C.S.G. was supported in part by postdoctoral fellowships from the National Institutes of Health and the Beckman Foundation. D.C.R. is an Investigator in the Howard Hughes Medical Institute.
Author Contributions Z.L. designed and performed the experiments in molecular biology, biochemistry, crystallography and the structure analysis. Z.L. and C.S.G. conducted the down-shock assays. C.S.G. was responsible for the protein reconstitution and electrophysiology. D.C.R. coordinated the project and contributed to the structure analysis. The manuscript was written by Z.L., C.S.G. and D.C.R.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-4 with Legends and Supplementary Table 1. (PDF 550 kb)
Supplementary Movie 1
This movie file shows proposed two-step helix pivoting model for SaMscL gating. (MOV 1680 kb)
Rights and permissions
About this article
Cite this article
Liu, Z., Gandhi, C. & Rees, D. Structure of a tetrameric MscL in an expanded intermediate state. Nature 461, 120–124 (2009). https://doi.org/10.1038/nature08277
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08277
This article is cited by
-
Elucidating a role for the cytoplasmic domain in the Mycobacterium tuberculosis mechanosensitive channel of large conductance
Scientific Reports (2018)
-
Architecture and Function of Mechanosensitive Membrane Protein Lattices
Scientific Reports (2016)
-
Architecture of the mammalian mechanosensitive Piezo1 channel
Nature (2015)
-
MscL: channeling membrane tension
Pflügers Archiv - European Journal of Physiology (2015)
-
Toward Understanding Protocell Mechanosensation
Origins of Life and Evolution of Biospheres (2011)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.