Abstract
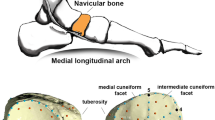
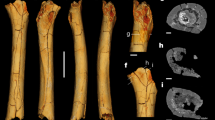
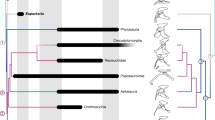
Homo floresiensis is an endemic hominin species that occupied Liang Bua, a limestone cave on Flores in eastern Indonesia, during the Late Pleistocene epoch1,2. The skeleton of the type specimen (LB1) of H. floresiensis includes a relatively complete left foot and parts of the right foot3. These feet provide insights into the evolution of bipedalism and, together with the rest of the skeleton, have implications for hominin dispersal events into Asia. Here we show that LB1’s foot is exceptionally long relative to the femur and tibia, proportions never before documented in hominins but seen in some African apes. Although the metatarsal robusticity sequence is human-like and the hallux is fully adducted, other intrinsic proportions and pedal features are more ape-like. The postcranial anatomy of H. floresiensis is that of a biped1,2,3, but the unique lower-limb proportions and surprising combination of derived and primitive pedal morphologies suggest kinematic and biomechanical differences from modern human gait. Therefore, LB1 offers the most complete glimpse of a bipedal hominin foot that lacks the full suite of derived features characteristic of modern humans and whose mosaic design may be primitive for the genus Homo. These new findings raise the possibility that the ancestor of H. floresiensis was not Homo erectus but instead some other, more primitive, hominin whose dispersal into southeast Asia is still undocumented.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brown, P. et al. A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature 431, 1055–1061 (2004)
Morwood, M. J. et al. Further evidence for small-bodied hominins from the Late Pleistocene of Flores, Indonesia. Nature 437, 1012–1017 (2005)
Jungers, W. L. et al. Descriptions of the lower limb skeleton of Homo floresiensis . J. Hum. Evol. 10.1016/j.jhevol.2008.08.014 (in the press)
Zollikofer, C. P. E. et al. Virtual cranial reconstruction of Sahelanthropus tchadensis . Nature 434, 755–759 (2005)
Richmond, B. G. & Jungers, W. L. Orrorin tugenensis femoral morphology and the evolution of hominin bipedalism. Science 319, 1662–1665 (2008)
Lordkipanidze, D. et al. Postcranial evidence from early Homo from Dmanisi, Georgia. Nature 449, 305–310 (2007)
White, T. D. & Suwa, G. Hominid footprints at Laetoli: facts and interpretations. Am. J. Phys. Anthropol. 72, 485–514 (1987)
Winter, D. A. Foot trajectory in human gait: a precise and multifactorial motor control task. Phys. Ther. 72, 45–53 (1992)
Jungers, W. L. & Stern, J. T. Jr. Body proportions, skeletal allometry and locomotion in the Hadar hominids: a reply to Wolpoff. J. Hum. Evol. 12, 673–684 (1983)
Archibald, J. D., Lovejoy, C. O. & Heiple, K. G. Implications of relative robusticity in the Olduvai metatarsus. Am. J. Phys. Anthropol. 37, 93–96 (1972)
Susman, R. L. & Brain, T. M. New first metatarsal (SKX 5017) from Swartkrans and the gait of Paranthropus robustus . Am. J. Phys. Anthropol. 77, 7–15 (1988)
Rodgers, M. M. & Cavanagh, P. R. Pressure distribution in Morton’s foot structure. Med. Sci. Sports Exerc. 21, 23–28 (1989)
Wallace, I. J. et al. The bipedalism of the Dmanisi hominins: pidgeon-toed early Homo? Am. J. Phys. Anthropol. 136, 375–378 (2008)
Susman, R. L., Stern, J. T. & Jungers, W. L. Arboreality and bipedality in the Hadar hominids. Folia Primatol. (Basel) 43, 113–156 (1984)
Bramble, D. M. & Lieberman, D. E. Endurance running and the evolution of Homo . Nature 432, 345–352 (2004)
Rolian, C. et al. Walking, running and the evolution of short toes in humans. J. Exp. Biol. 212, 713–721 (2009)
Harcourt-Smith, W. E. H. & Aiello, L. C. Fossils, feet, and the evolution of bipedal locomotion. J. Anat. 204, 403–416 (2004)
Vereecke, E. et al. Dynamic plantar pressure distribution during terrestrial locomotion of bonobos (Pan paniscus). Am. J. Phys. Anthropol. 120, 373–383 (2003)
Gebo, D. L. & Schwartz, G. T. Foot bones from Omo: implications for hominid evolution. Am. J. Phys. Anthropol. 129, 499–511 (2006)
Rhoads, J. G. & Trinkaus, E. Morphometrics of the Neandertal talus. Am. J. Phys. Anthropol. 46, 29–44 (1977)
Bojsen-Møller, F. Calcaneocuboid joint and stability of the longitudinal arch of the foot at high and low gear push off. J. Anat. 129, 165–176 (1979)
Lewis, O. J. Functional Morphology of the Evolving Hand and Foot 328–333 (Oxford Univ. Press, 1989)
Argue, D., Donlon, D., Groves, C. & Wright, R. Homo floresiensis: microcephalic, pygmoid, Australopithecus or Homo? J. Hum. Evol. 51, 360–374 (2006)
Falk, D. et al. Brain shape in human microcephalics and Homo floresiensis . Proc. Natl Acad. Sci. USA 104, 2513–2518 (2007)
Larson, S. G. et al. Homo floresiensis and the evolution of the hominin shoulder. J. Hum. Evol. 53, 718–731 (2007)
Tocheri, M. W. et al. The primitive wrist of Homo floresiensis and its implications for hominin evolution. Science 317, 1743–1745 (2007)
Morwood, M. J., O’Sullivan, P. B., Aziz, F. & Raza, A. Fission-track age of stone tools and fossils on the east Indonesian island of Flores. Nature 392, 173–176 (1998)
Lyras, G. S. et al. The origin of Homo floresiensis and its relation to evolutionary processes under isolation. Anthropol. Sci. advance online publication 10.1537/ase.080411 (1 August 2008)
Migliano, A. B., Vinicius, L. & Lahr, M. M. Life history trade-offs explain the evolution of human pygmies. Proc. Natl Acad. Sci. USA 104, 20216–20219 (2007)
Dennell, R. & Roebroeks, W. An Asian perspective on early human dispersal from Africa. Nature 438, 1099–1104 (2005)
Acknowledgements
This work was supported by grants from the Australian Research Council, the National Geographic Society, the Wenner-Gren Foundation for Anthropological Research, the Wellcome Trust and the Leakey Foundation. We also thank our colleagues Jatmiko, E. Wahyu Saptomo and T. Djubiantono for their encouragement and assistance in the excavations.
Author Contributions W.L.J., W.E.H.H.-S., R.E.W., M.W.T., M.J.M. and S.G.L. undertook the analyses of the pedal remains and wrote the paper; T.S. and R.A.D. were responsible for the Liang Bua excavations and assisted with data collection and analyses.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-6 with Legends, Supplementary Tables 1-3 and Supplementary References. (PDF 509 kb)
Rights and permissions
About this article
Cite this article
Jungers, W., Harcourt-Smith, W., Wunderlich, R. et al. The foot of Homo floresiensis. Nature 459, 81–84 (2009). https://doi.org/10.1038/nature07989
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07989
This article is cited by
-
Morphological and evolutionary insights into the keystone element of the human foot’s medial longitudinal arch
Communications Biology (2023)
-
Characterising the stone artefact raw materials at Liang Bua, Indonesia
Journal of Paleolithic Archaeology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.