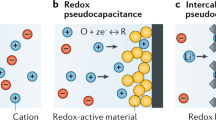
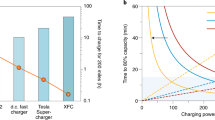
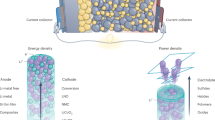
Abstract
The storage of electrical energy at high charge and discharge rate is an important technology in today’s society, and can enable hybrid and plug-in hybrid electric vehicles and provide back-up for wind and solar energy. It is typically believed that in electrochemical systems very high power rates can only be achieved with supercapacitors, which trade high power for low energy density as they only store energy by surface adsorption reactions of charged species on an electrode material1,2,3. Here we show that batteries4,5 which obtain high energy density by storing charge in the bulk of a material can also achieve ultrahigh discharge rates, comparable to those of supercapacitors. We realize this in LiFePO4 (ref. 6), a material with high lithium bulk mobility7,8, by creating a fast ion-conducting surface phase through controlled off-stoichiometry. A rate capability equivalent to full battery discharge in 10–20 s can be achieved.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Conway, B. E. Transition from supercapacitor to battery behavior in electrochemical energy-storage. J. Electrochem. Soc. 138, 1539–1548 (1991)
Arico, A. S., Bruce, P., Scrosati, B., Tarascon, J. M. & Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nature Mater. 4, 366–377 (2005)
Amatucci, G. G., Badway, F., Du Pasquier, A. & Zheng, T. An asymmetric hybrid nonaqueous energy storage cell. J. Electrochem. Soc. 148, A930–A939 (2001)
Tarascon, J. M. & Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001)
Reed, J. & Ceder, G. Role of electronic structure in the susceptibility of metastable transition-metal oxide structures to transformation. Chem. Rev. 104, 4513–4533 (2004)
Padhi, A. K., Nanjundaswamy, K. S. & Goodenough, J. B. Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J. Electrochem. Soc. 144, 1188–1194 (1997)
Islam, M. S., Driscoll, D. J., Fisher, C. A. J. & Slater, P. R. Atomic-scale investigation of defects, dopants, and lithium transport in the LiFePO4 olivine-type battery material. Chem. Mater. 17, 5085–5092 (2005)
Morgan, D., Van der Ven, A. & Ceder, G. Li conductivity in LixMPO4 (M = Mn, Fe, Co, Ni) olivine materials. Electrochem. Solid State Lett. 7, A30–A32 (2004)
Chung, S. Y., Bloking, J. T. & Chiang, Y. M. Electronically conductive phospho-olivines as lithium storage electrodes. Nature Mater. 1, 123–128 (2002)
Ravet, N. C. Y., Magnan, J. F., Besner, S., Gauthier, M. & Armand, M. Electroactivity of natural and synthetic triphylite. J. Power Sources 97–8, 503–507 (2001)
Herle, P. S., Ellis, B., Coombs, N. & Nazar, L. F. Nano-network electronic conduction in iron and nickel olivine phosphates. Nature Mater. 3, 147–152 (2004)
Delacourt, C., Poizot, P., Levasseur, S. & Masquelier, C. Size effects on carbon-free LiFePO4 powders. Electrochem. Solid State Lett. 9, A352–A355 (2006)
Kim, D. H. & Kim, J. Synthesis of LiFePO4 nanoparticles in polyol medium and their electrochemical properties. Electrochem. Solid State Lett. 9, A439–A442 (2006)
Chen, G. Y., Song, X. Y. & Richardson, T. J. Electron microscopy study of the LiFePO4 to FePO4 phase transition. Electrochem. Solid State Lett. 9, A295–A298 (2006)
Wang, B., Kwak, B. S., Sales, B. C. & Bates, J. B. Ionic conductivities and structure of lithium phosphorus oxynitride glasses. J. Non-Cryst. Solids 183, 297–306 (1995)
Sayer, M. & Mansingh, A. Transport properties of semiconducting phosphate glasses. Phys. Rev. B 6, 4629–4643 (1972)
Mogus-Milankovic, A., Santic, A., Karbulut, M. & Day, D. E. Study of electrical properties of MoO3-Fe2O3-P2O5 and SrO-Fe2O3-P2O5 glasses by impedance spectroscopy. II. J. Non-Cryst. Solids 330, 128–141 (2003)
Zhou, H. S., Li, D. L., Hibino, M. & Honma, I. A self-ordered, crystalline-glass, mesoporous nanocomposite for use as a lithium-based storage device with both high power and high energy densities. Angew. Chem. Int. Edn Engl. 44, 797–802 (2005)
Ong, S. P., Wang, L., Kang, B. & Ceder, G. Li-Fe-P-O2 phase diagram from first principles calculations. Chem. Mater. 20, 1798–1807 (2008)
Martin, S. W. Ionic-conduction in phosphate-glasses. J. Am. Ceram. Soc. 74, 1767–1784 (1991)
Sobha, K. C. & Rao, K. J. Investigation of phosphate glasses with the general formula AxByP3O12 where A = Li, Na or K and B = Fe, Ga, Ti, Ge, V or Nb. J. Non-Cryst. Solids 201, 52–65 (1996)
Kim, D.-K. et al. Effect of synthesis conditions on the properties of LiFePO4 for secondary lithium batteries. J. Power Sources 159, 237–240 (2006)
Ellis, B. et al. Nanostructured materials for lithium-ion batteries: Surface conductivity vs. bulk ion/electron transport. Faraday Discuss. 134, 119–141 (2007)
Rho, Y. H., Nazar, L. F., Perry, L. & Ryan, D. Surface chemistry of LiFePO4 studied by Mossbauer and X-ray photoelectron spectroscopy and its effect on electrochemical properties. J. Electrochem. Soc. 154, A283–A289 (2007)
Padhi, A. K., Nanjundaswamy, K. S., Masquelier, C., Okada, S. & Goodenough, J. B. Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates. J. Electrochem. Soc. 144, 1609–1613 (1997)
Morgan, W. E., Stec, W. J. & Vanwazer, J. R. Inner-orbital photoelectron spectroscopy of alkali-metal halides, perchlorates, phosphates, and pyrophosphates. J. Am. Chem. Soc. 95, 751–755 (1973)
Okubo, M. et al. Nanosize effect on high-rate Li-ion intercalation in LiCoO2 electrode. J. Am. Chem. Soc. 129, 7444–7452 (2007)
Wang, L., Zhou, F., Meng, Y. S. & Ceder, G. First-principles study of surface properties of LiFePO4: Surface energy, structure, Wulff shape, and surface redox potential. Phys. Rev. B 76, 165435 (2007)
Dominko, R., Gaberscek, M., Bele, A., Mihailovic, D. & Jamnik, J. Carbon nanocoatings on active materials for Li-ion batteries. J. Eur. Ceram. Soc. 27, 909–913 (2007)
Gaberscek, M., Dominko, R., Bele, M., Remskar, M. & Jamnik, J. Mass and charge transport in hierarchically organized storage materials. Example: Porous active materials with nanocoated walls of pores. Solid State Ionics 177, 3015–3022 (2006)
Acknowledgements
B.K. thanks D. S. Yun and Y. Zhang for the help with transmission electron microscope measurements and K. Kang and Y. S. Meng for experimental help and discussions. Support from the US National Science Foundation through the Materials Research Science and Engineering Centers programme and the Batteries for Advanced Transportation Program of the US Department of Energy is gratefully acknowledged.
Author Contributions B.K. performed the experiments and G.C. supervised and analysed the work.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Notes, Supplementary Figures S1-S6 with Legends, Supplementary Table 1, Supplementary Methods and Supplementary References. (PDF 620 kb)
Rights and permissions
About this article
Cite this article
Kang, B., Ceder, G. Battery materials for ultrafast charging and discharging. Nature 458, 190–193 (2009). https://doi.org/10.1038/nature07853
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07853
This article is cited by
-
Origin of fast charging in hard carbon anodes
Nature Energy (2024)
-
Learning heterogeneous reaction kinetics from X-ray videos pixel by pixel
Nature (2023)
-
Li iontronics in single-crystalline T-Nb2O5 thin films with vertical ionic transport channels
Nature Materials (2023)
-
SrTiO3-modified CaCu3Ti4O12 ceramics with low dielectric loss and excellent temperature stability for X8P capacitors
Journal of Materials Science: Materials in Electronics (2023)
-
P(VDF-TrFE)/ZnO nanocomposite synthesized by electrospinning: effect of ZnO nanofiller on physical, mechanical, thermal, rheological and piezoelectric properties
Polymer Bulletin (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.