Abstract
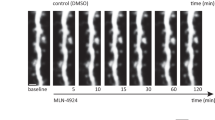
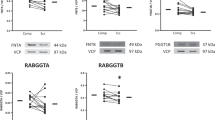
Palmitoylation regulates diverse aspects of neuronal protein trafficking and function. Here a global characterization of rat neural palmitoyl-proteomes identifies most of the known neural palmitoyl proteins—68 in total, plus more than 200 new palmitoyl-protein candidates, with further testing confirming palmitoylation for 21 of these candidates. The new palmitoyl proteins include neurotransmitter receptors, transporters, adhesion molecules, scaffolding proteins, as well as SNAREs and other vesicular trafficking proteins. Of particular interest is the finding of palmitoylation for a brain-specific Cdc42 splice variant. The palmitoylated Cdc42 isoform (Cdc42-palm) differs from the canonical, prenylated form (Cdc42-prenyl), both with regard to localization and function: Cdc42-palm concentrates in dendritic spines and has a special role in inducing these post-synaptic structures. Furthermore, assessing palmitoylation dynamics in drug-induced activity models identifies rapidly induced changes for Cdc42 as well as for other synaptic palmitoyl proteins, suggesting that palmitoylation may participate broadly in the activity-driven changes that shape synapse morphology and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Huang, K. & El-Husseini, A. Modulation of neuronal protein trafficking and function by palmitoylation. Curr. Opin. Neurobiol. 15, 527–535 (2005)
Resh, M. D. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci. STKE 2006, re14 (2006)
Smotrys, J. E. & Linder, M. E. Palmitoylation of intracellular signaling proteins: regulation and function. Annu. Rev. Biochem. 73, 559–587 (2004)
Linder, M. E. & Deschenes, R. J. Palmitoylation: policing protein stability and traffic. Nature Rev. Mol. Cell Biol. 8, 74–84 (2007)
El-Husseini, A. E.-D. et al. Synaptic strength regulated by palmitate cycling on PSD-95. Cell 108, 849–863 (2002)
Roth, A. F. et al. Global analysis of protein palmitoylation in yeast. Cell 125, 1003–1013 (2006)
Drisdel, R. C. & Green, W. N. Labeling and quantifying sites of protein palmitoylation. Biotechniques 36, 276–285 (2004)
Link, A. J. et al. Direct analysis of protein complexes using mass spectrometry. Nature Biotechnol. 17, 676–682 (1999)
Liu, H., Sadygov, R. G. & Yates, J. R. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 76, 4193–4201 (2004)
Wan, J. et al. Palmitoylated proteins: purification and identification. Nature Protoc. 2, 1573–1584 (2007)
Hayashi, T., Rumbaugh, G. & Huganir, R. L. Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron 47, 709–723 (2005)
Nishimura, T. et al. Role of numb in dendritic spine development with a Cdc42 GEF intersectin and EphB2. Mol. Biol. Cell 17, 1273–1285 (2006)
Negishi, M. & Katoh, H. Rho family GTPases and dendrite plasticity. Neuroscientist 11, 187–191 (2005)
Choi, J. et al. Regulation of dendritic spine morphogenesis by insulin receptor substrate 53, a downstream effector of Rac1 and Cdc42 small GTPases. J. Neurosci. 25, 869–879 (2005)
Scott, E. K., Reuter, J. E. & Luo, L. Small GTPase Cdc42 is required for multiple aspects of dendritic morphogenesis. J. Neurosci. 23, 3118–3123 (2003)
Nakazawa, T. et al. p250GAP, a novel brain-enriched GTPase-activating protein for Rho family GTPases, is involved in the N-methyl-d-aspartate receptor signaling. Mol. Biol. Cell 14, 2921–2934 (2003)
Wilson, A. L. et al. Prenylation of Rab8 GTPase by type I and type II geranylgeranyl transferases. Biochem. J. 333, 497–504 (1998)
Marks, P. W. & Kwiatkowski, D. J. Genomic organization and chromosomal location of murine Cdc42. Genomics 38, 13–18 (1996)
Kreis, P. et al. The p21-activated kinase 3 implicated in mental retardation regulates spine morphogenesis through a Cdc42-dependent pathway. J. Biol. Chem. 282, 21497–21506 (2007)
Node-Langlois, R., Muller, D. & Boda, B. Sequential implication of the mental retardation proteins ARHGEF6 and PAK3 in spine morphogenesis. J. Cell Sci. 119, 4986–4993 (2006)
Wegner, A. M. et al. N-wasp and the arp2/3 complex are critical regulators of actin in the development of dendritic spines and synapses. J. Biol. Chem. 283, 15912–15920 (2008)
Ethell, I. M. & Pasquale, E. B. Molecular mechanisms of dendritic spine development and remodeling. Prog. Neurobiol. 75, 161–205 (2005)
Hering, H., Lin, C. C. & Sheng, M. Lipid rafts in the maintenance of synapses, dendritic spines, and surface AMPA receptor stability. J. Neurosci. 23, 3262–3271 (2003)
Fischer, M. et al. Rapid actin-based plasticity in dendritic spines. Neuron 20, 847–854 (1998)
Ehlers, M. D. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nature Neurosci. 6, 231–242 (2003)
Kirov, S. A. & Harris, K. M. Dendrites are more spiny on mature hippocampal neurons when synapses are inactivated. Nature Neurosci. 2, 878–883 (1999)
McKinney, R. A. et al. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nature Neurosci. 2, 44–49 (1999)
Soriano, F. X. et al. Preconditioning doses of NMDA promote neuroprotection by enhancing neuronal excitability. J. Neurosci. 26, 4509–4518 (2006)
Halpain, S., Hipolito, A. & Saffer, L. Regulation of F-actin stability in dendritic spines by glutamate receptors and calcineurin. J. Neurosci. 18, 9835–9844 (1998)
Fannjiang, Y. et al. BAK alters neuronal excitability and can switch from anti- to pro-death function during postnatal development. Dev. Cell 4, 575–585 (2003)
Chen, L. & Toth, M. Fragile X mice develop sensory hyperreactivity to auditory stimuli. Neuroscience 103, 1043–1050 (2001)
Mollner, S., Beck, K. & Pfeuffer, T. Acylation of adenylyl cyclase catalyst is important for enzymic activity. FEBS Lett. 371, 241–244 (1995)
Acknowledgements
This paper is dedicated to the memory of our friend and colleague, Alaa El-Husseini, whose ideas about palmitoylation and plasticity inspired this work (deceased 23 Dec 2007). We thank J. Levinson, M.-F. Lise, C. Jiang and E. Yu for technical assistance. This work was supported by grants to A.E.-H. from the Canadian Institutes for Health Research (CIHR) (A.E.-H., 20R90479 and 20R91909), the Michael Smith foundation for Health Research (A.E.-H., 20R52464), the EJLB Foundation and Neuroscience Canada (A.E.-H., 20R61933), as well as from grants from the National Institutes of Health to N.G.D. (GM65525), J.R.Y. (RR011823) and W.N.G. (NS043782, DA13602 and DA019695), and the Peter F. McManus Trust. H.T. was supported by a research fellowship from the Uehara Memorial Foundation. We thank L. Raymond, Y. T. Wang, K. Gerrow, R. Hines, M. Prior and I. Papanayotou for comments on manuscript.
Author Contributions R.K. and J.W. are co-first authors. R.K. was responsible for assessing candidate palmitoyl-protein palmitoylation, siRNA knockdown effects in neurons, and activity-dependent palmitoylation changes. J.W. was responsible for the ABE purifications of samples used for western blotting and mass spectrometry analysis, and for the quantitative northern analysis. P.A. and H.T. analysed filopodia and spine changes in transfected neurons. K.H. analysed palmitoylated proteins using an ABE assay. A.O.B., J.X.T. and J.R.Y. performed the mass spectrometry. N.G.D. analysed, assembled and interpreted the mass spectral data. R.C.D., R.M. and W.N.G. contributed to analysis of some of the palmitoylated proteins. A.F.R. constructed plasmids, particularly those used for the siRNA analysis and rescue. The original co-corresponding authors, A.E.-H. and N.G.D., provided hypothesis development, experimental design input, data interpretation and co-wrote the manuscript. With the passing of A.E.-H., N.G.D. supervised the experimental analyses and rewriting required for the revised manuscript.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-12 with Legends, Supplementary Methods, Supplementary Discussions and Supplementary References. (PDF 8348 kb)
Supplementary Tables
This file contains Supplementary Tables 1-6 with Supplementary Data. (PDF 1726 kb)
Rights and permissions
About this article
Cite this article
Kang, R., Wan, J., Arstikaitis, P. et al. Neural palmitoyl-proteomics reveals dynamic synaptic palmitoylation. Nature 456, 904–909 (2008). https://doi.org/10.1038/nature07605
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature07605
This article is cited by
-
Reduction of DHHC5-mediated beclin 1 S-palmitoylation underlies autophagy decline in aging
Nature Structural & Molecular Biology (2024)
-
Global profiling of protein S-palmitoylation in the second-generation merozoites of Eimeria tenella
Parasitology Research (2024)
-
Limb-Clasping Response in NMDA Receptor Palmitoylation-Deficient Mice
Molecular Neurobiology (2024)
-
Prolonged contextual fear memory in AMPA receptor palmitoylation-deficient mice
Neuropsychopharmacology (2022)
-
Crosstalk of Synapsin1 palmitoylation and phosphorylation controls the dynamicity of synaptic vesicles in neurons
Cell Death & Disease (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.