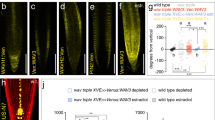
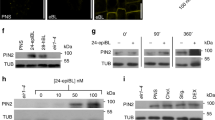
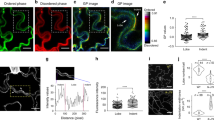
Abstract
Dynamically polarized membrane proteins define different cell boundaries and have an important role in intercellular communication—a vital feature of multicellular development. Efflux carriers for the signalling molecule auxin from the PIN family1 are landmarks of cell polarity in plants and have a crucial involvement in auxin distribution-dependent development including embryo patterning, organogenesis and tropisms2,3,4,5,6,7. Polar PIN localization determines the direction of intercellular auxin flow8, yet the mechanisms generating PIN polarity remain unclear. Here we identify an endocytosis-dependent mechanism of PIN polarity generation and analyse its developmental implications. Real-time PIN tracking showed that after synthesis, PINs are initially delivered to the plasma membrane in a non-polar manner and their polarity is established by subsequent endocytic recycling. Interference with PIN endocytosis either by auxin or by manipulation of the Arabidopsis Rab5 GTPase pathway prevents PIN polarization. Failure of PIN polarization transiently alters asymmetric auxin distribution during embryogenesis and increases the local auxin response in apical embryo regions. This results in ectopic expression of auxin pathway-associated root-forming master regulators in embryonic leaves and promotes homeotic transformation of leaves to roots. Our results indicate a two-step mechanism for the generation of PIN polar localization and the essential role of endocytosis in this process. It also highlights the link between endocytosis-dependent polarity of individual cells and auxin distribution-dependent cell fate establishment for multicellular patterning.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Petrasek, J. et al. PIN proteins perform a rate-limiting function in cellular auxin efflux. Science 312, 914–918 (2006)
Galweiler, L. et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230 (1998)
Friml, J. et al. Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis . Nature 426, 147–153 (2003)
Benkova, E. et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115, 591–602 (2003)
Reinhardt, D. et al. Regulation of phyllotaxis by polar auxin transport. Nature 426, 255–260 (2003)
Heisler, M. G. et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr. Biol. 15, 1899–1911 (2005)
Luschnig, C., Gaxiola, R. A., Grisafi, P. & Fink, G. R. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 12, 2175–2187 (1998)
Wisniewska, J. et al. Polar PIN localization directs auxin flow in plants. Science 312, 883 (2006)
Stepanova, A. N. et al. TAA1-mediated auxin biosynthesis is essential for hormone crosstalk and plant development. Cell 133, 177–191 (2008)
Zhao, Y. The role of local biosynthesis of auxin and cytokinin in plant development. Curr. Opin. Plant Biol. 11, 16–22 (2008)
Tanaka, H., Dhonukshe, P., Brewer, P. B. & Friml, J. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell. Mol. Life Sci. 63, 2738–2754 (2006)
Dhonukshe, P. et al. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis . Curr. Biol. 17, 520–527 (2007)
Geldner, N., Friml, J., Stierhof, Y. D., Jurgens, G. & Palme, K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413, 425–428 (2001)
Kleine-Vehn, J. et al. ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis . Curr. Biol. 18, 526–531 (2008)
Friml, J., Wisniewska, J., Benkova, E., Mendgen, K. & Palme, K. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis . Nature 415, 806–809 (2002)
Friml, J. et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science 306, 862–865 (2004)
Michniewicz, M. et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 130, 1044–1056 (2007)
Men, S. et al. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nature Cell Biol. 10, 237–244 (2008)
Mostov, K., Su, T. & ter Beest, M. Polarized epithelial membrane traffic: conservation and plasticity. Nature Cell Biol. 5, 287–293 (2003)
Paciorek, T. et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 435, 1251–1256 (2005)
Bucci, C. et al. The small GTPase rab5 functions as a regulatory factor in the early endocytic pathway. Cell 70, 715–728 (1992)
Ueda, T., Uemura, T., Sato, M. H. & Nakano, A. Functional differentiation of endosomes in Arabidopsis cells. Plant J. 40, 783–789 (2004)
Friml, J. et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis . Cell 108, 661–673 (2002)
Blilou, I. et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44 (2005)
Swarup, R. et al. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 15, 2648–2653 (2001)
Kleine-Vehn, J., Dhonukshe, P., Swarup, R., Bennett, M. & Friml, J. Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell 18, 3171–3181 (2006)
Weijers, D. et al. An Arabidopsis Minute-like phenotype caused by a semi-dominant mutation in a RIBOSOMAL PROTEIN S5 gene. Development 128, 4289–4299 (2001)
Benfey, P. N. & Chua, N. H. The cauliflower mosaic virus 35S promoter: combinatorial regulation of transcription in plants. Science 250, 959–966 (1990)
Aida, M. et al. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell 119, 109–120 (2004)
Sarkar, A. K. et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature 446, 811–814 (2007)
Dhonukshe, P. et al. Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev. Cell 10, 137–150 (2006)
Xu, J. et al. A molecular framework for plant regeneration. Science 311, 385–388 (2006)
Xu, J. & Scheres, B. Dissection of Arabidopsis ADP-RIBOSYLATION FACTOR 1 function in epidermal cell polarity. Plant Cell 17, 525–536 (2005)
Jaillais, Y. et al. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell 130, 1057–1070 (2007)
Geldner, N. et al. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112, 219–230 (2003)
Richter, S. et al. Functional diversification of closely related ARF-GEFs in protein secretion and recycling. Nature 448, 488–492 (2007)
Dettmer, J., Hong-Hermesdorf, A., Stierhof, Y. D. & Schumacher, K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis . Plant Cell 18, 715–730 (2006)
Swarup, R. et al. Structure-function analysis of the presumptive Arabidopsis auxin permease AUX1. Plant Cell 16, 3069–3083 (2004)
Sabatini, S. et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99, 463–472 (1999)
Goh, T. et al. VPS9a, the common activator for two distinct types of Rab5 GTPases, is essential for the development of Arabidopsis thaliana. Plant Cell 19, 3504–3515 (2007)
Abas, L. et al. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nature Cell Biol. 8, 249–256 (2006)
Lam, S. K. et al. Rice SCAMP1 defines clathrin-coated, trans-golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell 19, 296–319 (2007)
Langhans, M. et al. Immunolocalization of plasma-membrane H+-ATPase and tonoplast-type pyrophosphatase in the plasma membrane of the sieve element-companion cell complex in the stem of Ricinus communis L. Planta 213, 11–19 (2001)
Movafeghi, A., Happel, N., Pimpl, P., Tai, G. H. & Robinson, D. G. Arabidopsis Sec21p and Sec23p homologs. Probable coat proteins of plant COP-coated vesicles. Plant Physiol. 119, 1437–1446 (1999)
Acknowledgements
We thank M. Bennett, T. Gaude, L. Jiang, G. Jürgens, C. Luschnig, E. Meywerowitz, W. Michalke, R. Offringa, D. Robinson, K. Schumacher, J. Wiedenmann and D. Weijers for sharing published material and RIKEN, SALK and NASC Arabidopsis stock centres for providing mutant lines and the RNAi construct. We thank W. Muller for assistance with SEM and F. Kindt and R. Leito for photography. We acknowledge the Center for Plant Molecular Biology (ZMBP), University of Tübingen, Germany for the facilities during the initial phase of the project. This work was supported by VolkswagenStiftung (P.D. and J.F.), EMBO Long Term Fellowship and Netherlands Organization for Scientific Research (NWO)-VENI grant (P.D.), EMBO Young Investigator Program and Odysseus program of FWO (J.F.), HFSP fellowship (H.T.), EMBO Long Term Fellowship and HFSP fellowship (A.P.M.), EMBO Long Term Fellowship (K.P.), NWO-VIDI grant (I.B.), HFSP fellowship and Swiss National Science Foundation (N.G.), HHMI, USDA and NIH (J.C.), NWO-Spinoza award (B.S.) and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (T.U. and A.N.).
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Information
This file contains Supplementary Results, Supplementary References, Supplementary Figures 1-14 with Legends and Supplementary Table 1. (PDF 15176 kb)
Rights and permissions
About this article
Cite this article
Dhonukshe, P., Tanaka, H., Goh, T. et al. Generation of cell polarity in plants links endocytosis, auxin distribution and cell fate decisions. Nature 456, 962–966 (2008). https://doi.org/10.1038/nature07409
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07409
This article is cited by
-
Inconsistencies in the root biology terminology: Let’s communicate better
Plant and Soil (2022)
-
An essential role for Arabidopsis Trs33 in cell growth and organization in plant apical meristems
Plant Cell Reports (2020)
-
SlPIN1 regulates auxin efflux to affect flower abscission process
Scientific Reports (2017)
-
Cellular mechanisms for cargo delivery and polarity maintenance at different polar domains in plant cells
Cell Discovery (2016)
-
Genetical genomics of Populus leaf shape variation
BMC Plant Biology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.