Abstract
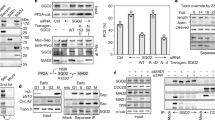
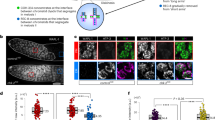
At the onset of anaphase, sister-chromatid cohesion is dissolved abruptly and irreversibly, ensuring that all chromosome pairs disjoin almost simultaneously. The regulatory mechanisms that generate this switch-like behaviour are unclear. Anaphase is initiated when a ubiquitin ligase, the anaphase-promoting complex (APC), triggers the destruction of securin, thereby allowing separase, a protease, to disrupt sister-chromatid cohesion1,2,3,4. Here we demonstrate that the cyclin-dependent kinase 1 (Cdk1)-dependent phosphorylation of securin near its destruction-box motif inhibits securin ubiquitination by the APC. The phosphatase Cdc14 reverses securin phosphorylation, thereby increasing the rate of securin ubiquitination. Because separase is known to activate Cdc14 (refs 5 and 6), our results support the existence of a positive feedback loop that increases the abruptness of anaphase. Consistent with this model, we show that mutations that disrupt securin phosphoregulation decrease the synchrony of chromosome segregation. Our results also suggest that coupling securin degradation with changes in Cdk1 and Cdc14 activities helps coordinate the initiation of sister-chromatid separation with changes in spindle dynamics.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nasmyth, K. Segregating sister genomes: the molecular biology of chromosome separation. Science 297, 559–565 (2002)
Peters, J. M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nature Rev. Mol. Cell Biol. 7, 644–656 (2006)
Thornton, B. R. & Toczyski, D. P. Precise destruction: an emerging picture of the APC. Genes Dev. 20, 3069–3078 (2006)
Sullivan, M. & Morgan, D. O. Finishing mitosis, one step at a time. Nature Rev. Mol. Cell Biol. 8, 894–903 (2007)
Stegmeier, F., Visintin, R. & Amon, A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell 108, 207–220 (2002)
Queralt, E., Lehane, C., Novak, B. & Uhlmann, F. Downregulation of PP2A(Cdc55) phosphatase by separase initiates mitotic exit in budding yeast. Cell 125, 719–732 (2006)
Agarwal, R., Tang, Z., Yu, H. & Cohen-Fix, O. Two distinct pathways for inhibiting pds1 ubiquitination in response to DNA damage. J. Biol. Chem. 278, 45027–45033 (2003)
Wang, H. et al. Pds1 phosphorylation in response to DNA damage is essential for its DNA damage checkpoint function. Genes Dev. 15, 1361–1372 (2001)
Blethrow, J. D., Tang, C., Deng, C. & Krutchinsky, A. N. Modular mass spectrometric tool for analysis of composition and phosphorylation of protein complexes. PLoS ONE 2, e358 (2007)
Hornig, N. C., Knowles, P. P., McDonald, N. Q. & Uhlmann, F. The dual mechanism of separase regulation by securin. Curr. Biol. 12, 973–982 (2002)
Agarwal, R. & Cohen-Fix, O. Phosphorylation of the mitotic regulator Pds1/securin by Cdc28 is required for efficient nuclear localization of Esp1/separase. Genes Dev. 16, 1371–1382 (2002)
King, R. W., Glotzer, M. & Kirschner, M. W. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol. Biol. Cell 7, 1343–1357 (1996)
Wan, J., Xu, H. & Grunstein, M. CDC14 of Saccharomyces cerevisiae . J. Biol. Chem. 267, 11274–11280 (1992)
Visintin, R. et al. The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol. Cell 2, 709–718 (1998)
Jaspersen, S. L., Charles, J. F. & Morgan, D. O. Inhibitory phosphorylation of the APC regulator Hct1 is controlled by the kinase Cdc28 and the phosphatase Cdc14. Curr. Biol. 9, 227–236 (1999)
Stegmeier, F. & Amon, A. Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu. Rev. Genet. 38, 203–232 (2004)
Ferrell, J. E. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14, 140–148 (2002)
Tyson, J. J., Chen, K. C. & Novak, B. Sniffers, buzzers, toggles and blinkers: dynamics of regulatory and signaling pathways in the cell. Curr. Opin. Cell Biol. 15, 221–231 (2003)
Straight, A. F., Marshall, W. F., Sedat, J. W. & Murray, A. W. Mitosis in living budding yeast: anaphase A but no metaphase plate. Science 277, 574–578 (1997)
Michaelis, C., Ciosk, R. & Nasmyth, K. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell 91, 35–45 (1997)
Wäsch, R. & Cross, F. APC-dependent proteolysis of the mitotic cyclin Clb2 is essential for mitotic exit. Nature 418, 556–562 (2002)
Higuchi, T. & Uhlmann, F. Stabilization of microtubule dynamics at anaphase onset promotes chromosome segregation. Nature 433, 171–176 (2005)
Pereira, G. & Schiebel, E. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science 302, 2120–2124 (2003)
Woodbury, E. L. & Morgan, D. O. Cdk and APC activities limit the spindle-stabilizing function of Fin1 to anaphase. Nature Cell Biol. 9, 106–112 (2007)
Parry, D. H., Hickson, G. R. & O’Farrell, P. H. Cyclin B destruction triggers changes in kinetochore behavior essential for successful anaphase. Curr. Biol. 13, 647–653 (2003)
Vig, B. K. Sequence of centromere separation: occurrence, possible significance, and control. Cancer Genet. Cytogenet. 8, 249–274 (1983)
Gerlich, D. et al. Global chromosome positions are transmitted through mitosis in mammalian cells. Cell 112, 751–764 (2003)
Carroll, C. W. & Morgan, D. O. Enzymology of the anaphase-promoting complex. Methods Enzymol. 398, 219–230 (2005)
Puig, O. et al. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods 24, 218–229 (2001)
Sage, D. et al. Automatic tracking of individual fluorescence particles: application to the study of chromosome dynamics. IEEE Trans. Image Process. 14, 1372–1383 (2005)
Acknowledgements
We thank: P. H. O’Farrell, A. D. Johnson and M. J. Sullivan for discussions; J. A. Ubersax, G. Goshima and O. Cohen-Fix for reagents; S. Foster, M. C. Rodrigo-Brenni, M. Enquist-Newman and the Morgan laboratory for help generating strains and reagents; J. M. Pedraza and A. van Oudenaarden for help with the model; K. S. Thorn and the University of California, San Francisco, Nikon Imaging Center for help with microscopy; and M. J. Sullivan and J. L. Feldman for reading the manuscript. This work was supported by funding from the National Institute of General Medical Sciences (D.O.M.), a grant from the Sandler Family Foundation (A.N.K.) and a fellowship from the National Science Foundation (L.J.H.).
Author Contributions L.J.H. designed, performed and analysed the experiments; A.N.K performed mass spectrometric analysis; D.O.M. provided guidance.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-9 with Legends and legends to Supplementary Movies 1-3 (PDF 1798 kb)
Supplementary Movie 1
The file contains Supplementary Movie 1 showing wild-type cell. (MOV 1197 kb)
Supplementary Movie 2
The file contains Supplementary Movie 2 showing securin-2A cell. (MOV 1058 kb)
Supplementary Movie 3
The file contains Supplementary Movie 3 showing securinΔ cell. (MOV 476 kb)
Rights and permissions
About this article
Cite this article
Holt, L., Krutchinsky, A. & Morgan, D. Positive feedback sharpens the anaphase switch. Nature 454, 353–357 (2008). https://doi.org/10.1038/nature07050
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07050
This article is cited by
-
The importance of CDC27 in cancer: molecular pathology and clinical aspects
Cancer Cell International (2021)
-
An NADPH oxidase regulates carbon metabolism and the cell cycle during root nodule symbiosis in common bean (Phaseolus vulgaris)
BMC Plant Biology (2021)
-
Mechanisms for the temporal regulation of substrate ubiquitination by the anaphase-promoting complex/cyclosome
Cell Division (2019)
-
Motif co-regulation and co-operativity are common mechanisms in transcriptional, post-transcriptional and post-translational regulation
Cell Communication and Signaling (2015)
-
Quantitative framework for ordered degradation of APC/C substrates
BMC Biology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.