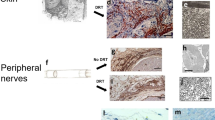
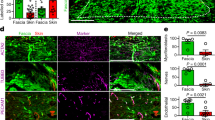
Abstract
The repair of wounds is one of the most complex biological processes that occur during human life. After an injury, multiple biological pathways immediately become activated and are synchronized to respond. In human adults, the wound repair process commonly leads to a non-functioning mass of fibrotic tissue known as a scar. By contrast, early in gestation, injured fetal tissues can be completely recreated, without fibrosis, in a process resembling regeneration. Some organisms, however, retain the ability to regenerate tissue throughout adult life. Knowledge gained from studying such organisms might help to unlock latent regenerative pathways in humans, which would change medical practice as much as the introduction of antibiotics did in the twentieth century.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Singer, A. J. & Clark, R. A. Cutaneous wound healing. N. Engl. J. Med. 341, 738–746 (1999).
Aarabi, S., Longaker, M. T. & Gurtner, G. C. Hypertrophic scar formation following burns and trauma: new approaches to treatment. PLoS Med. 4, e234 (2007).
Konigova, R. & Rychterova, V. Marjolin's ulcer. Acta Chir. Plast. 42, 91–94 (2000).
Trent, J. T. & Kirsner, R. S. Wounds and malignancy. Adv. Skin Wound Care 16, 31–34 (2003).
Colwell, A. S., Longaker, M. T. & Lorenz, H. P. Fetal wound healing. Front. Biosci. 8, s1240–s1248 (2003).
Gurtner, G. C., Callaghan, M. J. & Longaker, M. T. Progress and potential for regenerative medicine. Annu. Rev. Med. 58, 299–312 (2007).
National Heart, Lung, and Blood Institute. Morbidity & Mortality: 2002 Chart Book on Cardiovascular, Lung, and Blood Diseases (US Department of Health and Human Services, Bethesda, 2002).
Anderson, R. N. & Smith, B. L. Deaths: leading causes for 2001. Natl Vital Stat. Rep. 52 (9), 1–85 (2003).
Selman, M., King, T. E. & Pardo, A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern. Med. 134, 136–151 (2001).
Mescher, A. L. & Neff, A. W. Regenerative capacity and the developing immune system. Adv. Biochem. Eng. Biotechnol. 93, 39–66 (2005).
Klein, L. et al. Pharmacologic therapy for patients with chronic heart failure and reduced systolic function: review of trials and practical considerations. Am. J. Cardiol. 91, 18F–40F (2003).
Nichols, S. A., Dirks, W., Pearse, J. S. & King, N. Early evolution of animal cell signaling and adhesion genes. Proc. Natl Acad. Sci.USA 103, 12451–12456 (2006).
Adamska, M. et al. The evolutionary origin of hedgehog proteins. Curr. Biol. 17, R836–R837 (2007).
Adamska, M. et al. Wnt and TGF-β expression in the sponge Amphimedon queenslandica and the origin of metazoan embryonic patterning. PLoS ONE 2, e1031 (2007).
Adamska, M. et al. The evolutionary origin of hedgehog proteins. Curr. Biol. 17, R836–R837 (2007).
Woolner, S., Jacinto, A. & Martin, P. The small GTPase Rac plays multiple roles in epithelial sheet fusion — dynamic studies of Drosophila dorsal closure. Dev. Biol. 282, 163–173 (2005).
Samakovlis, C. et al. Genetic control of epithelial tube fusion during Drosophila tracheal development. Development 122, 3531–3536 (1996).This paper shows that the molecular machinery involved in dorsal closure in D. melanogaster embryos is also used during wound closure.
Wood, W. et al. Wound healing recapitulates morphogenesis in Drosophila embryos. Nature Cell Biol. 4, 907–912 (2002).
Woolley, K. & Martin, P. Conserved mechanisms of repair: from damaged single cells to wounds in multicellular tissues. Bioessays 22, 911–919 (2000).
Martin, P. & Parkhurst, S. M. Parallels between tissue repair and embryo morphogenesis. Development 131, 3021–3034 (2004).
Suzuki, M., Satoh, A., Ide, H. & Tamura, K. Nerve-dependent and -independent events in blastema formation during Xenopus froglet limb regeneration. Dev. Biol. 286, 361–375 (2005).
Brockes, J. P. & Kumar, A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nature Rev. Mol. Cell Biol. 3, 566–574 (2002).
Endo, T., Bryant, S. V. & Gardiner, D. M. A stepwise model system for limb regeneration. Dev. Biol. 270, 135–145 (2004).
Kumar, A., Godwin, J. W., Gates, P. B., Garza-Garcia, A. A. & Brockes, J. P. Molecular basis for the nerve dependence of limb regeneration in an adult vertebrate. Science 318, 772–777 (2007). This study found that the nerve dependence of limb regeneration results from nerves producing the protein nAG, which is a ligand for Prod 1 at the surface of blastemal cells, stimulating their proliferation.
Ánchez Alvarado, A. Planarian regeneration: its end is its beginning. Cell 124, 241–245 (2006).
Klapka, N. & Müller, H. W. Collagen matrix in spinal cord injury. J. Neurotrauma 23, 422 (2006).
Stichel, C. C. & Müller, H. W. The CNS lesion scar: new vistas on an old regeneration barrier. Cell Tissue Res. 294, 1–9 (1998).
Grose, R. & Werner, S. Wound-healing studies in transgenic and knockout mice. Mol. Biotechnol. 28, 147–166 (2004).
Martin, P. & Leibovich, S. J. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol. 15, 599–607 (2005). This review comprehensively synthesizes the voluminous literature on the role of the inflammatory process in wound repair, and the authors suggest that eliminating some physical components might ultimately improve tissue regeneration.
Martin, P. et al. Wound healing in the PU.1 null mouse — tissue repair is not dependent on inflammatory cells. Curr. Biol. 13, 1122–1128 (2003).
Werner, S. & Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 83, 835–870 (2003).
Galiano, R. D. et al. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 164, 1935–1947 (2004).
Bluff, J. E., Ferguson, M. W. J., O'Kane, S. & Ireland, G. Bone marrow-derived endothelial progenitor cells do not contribute significantly to new vessels during incisional wound healing. Exp. Hematol. 35, 500–506 (2007).
Opalenik, S. R. & Davidson, J. M. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J. 19, 1561–1563 (2005).
Werner, S., Krieg, T. & Smola, H. Keratinocyte–fibroblast interactions in wound healing. J. Invest. Dermatol. 127, 998–1008 (2007).
Szabowski, A. et al. c-Jun and JunB antagonistically control cytokine-regulated mesenchymal–epidermal interaction in skin. Cell 103, 745–755 (2000).
Lovvorn, H. N. et al. Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J. Pediatr. Surg. 34, 218–223 (1999).
Levenson, S. M. et al. The healing of rat skin wounds. Ann. Surg. 161, 293–308 (1965).
Rubin, G. M. et al. Comparative genomics of the eukaryotes. Science 287, 2204–2215 (2000).
Cole, J., Tsou, R., Wallace, K., Gibran, N. & Isik, F. Early gene expression profile of human skin to injury using high-density cDNA microarrays. Wound Repair Regen. 9, 360–370 (2001).
Cooper, L., Johnson, C., Burslem, F. & Martin, P. Wound healing and inflammation genes revealed by array analysis of 'macrophageless' PU.1 null mice. Genome Biol. 6, R5 (2005).
Chang, H. Y. et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2, E7 (2004).
Raja, K., Sivamani, M. S., Garcia, R. R. & Isseroff, R. R. Wound re-epithelialization: modulating keratinocyte migration in wound healing. Front. Biosci. 12, 2849–2868 (2007).
Chmielowiec, J. et al. c-Met is essential for wound healing in the skin. J. Cell Biol. 177, 151–162 (2007). This paper describes the essential role of the HGF receptor, MET, in wound re-epithelialization: cells deficient in this protein cannot contribute to the formation of a neo-epidermis.
Werner, S. et al. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science 266, 819–822 (1994). This study was the first to use genetically modified mice to study wound repair.
Braun, S., auf dem Keller, U., Steiling, H. & Werner, S. Fibroblast growth factors in epithelial repair and cytoprotection. Phil. Trans. R. Soc. Lond. B 359, 753–757 (2004).
Jameson, J. et al. A role for skin γδ T cells in wound repair. Science 296, 747–749 (2002).
Shirakata, Y. et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J. Cell Sci. 118, 2363–2370 (2005).
Schäfer, M. & Werner, S. Transcriptional control of wound repair. Annu. Rev. Cell Dev. Biol. 23, 69–92 (2007).
Li, G. et al. c-Jun is essential for organization of the epidermal leading edge. Dev. Cell 4, 865–877 (2003).
Sano, S. et al. Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. EMBO J. 18, 4657–4668 (1999).
Amendt, C., Mann, A., Schirmacher, P. & Blessing, M. Resistance of keratinocytes to TGFβ-mediated growth restriction and apoptosis induction accelerates re-epithelialization in skin wounds. J. Cell Sci. 115, 2189–2198 (2002).
Ashcroft, G. S. et al. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nature Cell Biol. 1, 260–266 (1999). This paper describes the inhibitory role of TGF- β -mediated signalling in the wound repair process.
Werner, S. & Alzheimer, C. Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine Growth Factor Rev. 17, 157–171 (2006).
Chernyavsky, A. I., Arredondo, J., Wess, J. R., Karlsson, E. & Grando, S. A. Novel signaling pathways mediating reciprocal control of keratinocyte migration and wound epithelialization through M3 and M4 muscarinic receptors. J. Cell Biol. 166, 261–272 (2004).
Pullar, C. E., Rizzo, A. & Isseroff, R. R. β-Adrenergic receptor antagonists accelerate skin wound healing: evidence for a catecholamine synthesis network in the epidermis. J. Biol. Chem. 281, 21225–21235 (2006).
Michalik, L. et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)α and PPARβ mutant mice. J. Cell Biol. 154, 799–814 (2001).
Icre, G., Wahli, W. & Michalik, L. Functions of the peroxisome proliferator-activated receptor (PPAR)α and β in skin homeostasis, epithelial repair, and morphogenesis. J. Invest. Dermatol. Symp. Proc. 11, 30–35 (2006).
Di-Poï, N., Tan, N. S., Michalik, L., Wahli, W. & Desvergne, B. Antiapoptotic role of PPARβ in keratinocytes via transcriptional control of the Akt1 signaling pathway. Mol. Cell 10, 721–733 (2002).
Zhao, M. et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-γ and PTEN. Nature 442, 457–460 (2006). This paper describes a crucial role for electrical signals in the re-epithelialization of wounds.
Claudinot, S. P., Nicolas, M., Oshima, H., Rochat, A. & Barrandon, Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl Acad. Sci. USA 102, 14677–14682 (2005).
Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T. T. & Lavker, R. M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102, 451–461 (2000).
Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104, 233–245 (2001).
Rochat, A., Kobayashi, K. & Barrandon, Y. Location of stem cells of human hair follicles by clonal analysis. Cell 76, 1063–1073 (1994).
Ito, M. et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature Med. 11, 1351–1354 (2005).
Levy, V., Lindon, C., Harfe, B. D. & Morgan, B. A. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev. Cell 9, 855–861 (2005).
Clayton, E. et al. A single type of progenitor cell maintains normal epidermis. Nature 446, 185–189 (2007).
Potten, C. S. & Booth, C. Keratinocyte stem cells: a commentary. J. Invest. Dermatol. 119, 888–899 (2002).
Barker, N. et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007).
Kiel, M. J. et al. Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature 449, 238–242 (2007).
Vauclair, S. et al. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev. Cell 13, 242–253 (2007).
Pearton, D. J., Yang, Y. & Dhouailly, D. Transdifferentiation of corneal epithelium into epidermis occurs by means of a multistep process triggered by dermal developmental signals. Proc. Natl Acad. Sci. USA 102, 3714–3719 (2005).
Nishida, K. et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 351, 1187–1196 (2004).
Mavilio, F. et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nature Med. 12, 1397–1402 (2006).
Metcalfe, F. & Ferguson, F. Skin stem and progenitor cells: using regeneration as a tissue-engineering strategy. Cell. Mol. Life Sci. 65, 24–32 (2008).
Metcalfe, A. D. & Ferguson, M. W. J. Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 4, 413–437 (2007).
Ito, M. et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320 (2007).
Reynolds, A. J., Lawrence, C., Cserhalmi-Friedman, P. B., Christiano, A. M. & Jahoda, C. A. Trans-gender induction of hair follicles. Nature 402, 33–34 (1999).
Fernandes, K. J. L. et al. A dermal niche for multipotent adult skin-derived precursor cells. Nature Cell Biol. 6, 1082–1093 (2004).
Wong, C. E. et al. Neural crest-derived cells with stem cell features can be traced back to multiple lineages in the adult skin. J. Cell Biol. 175, 1005–1015 (2006).
Blanpain, C., Lowry, W. E., Geoghegan, A., Polak, L. & Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648 (2004).
Lin, R. Y. et al. Exogenous transforming growth factor-β amplifies its own expression and induces scar formation in a model of human fetal skin repair. Ann. Surg. 222, 146–154 (1995).
Ghahary, A., Shen, Y. J., Scott, P. G., Gong, Y. & Tredget, E. E. Enhanced expression of mRNA for transforming growth factor-β, type I and type III procollagen in human post-burn hypertrophic scar tissues. J. Lab. Clin. Med. 122, 465–473 (1993).
Ferguson, M. W. & O'Kane, S. Scar-free healing: from embryonic mechanisms to adult therapeutic intervention. Phil. Trans. R. Soc. Lond. B 359, 839–850 (2004).
Arabi, S. et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 21, 3250–3261 (2007)
Lutolf, M. P. & Hubbell, J. A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature Biotechnol. 23, 47–55 (2005). This review comprehensively describes state-of-the-art synthetic biomaterials for guiding cell differentiation and tissue regeneration.
Acknowledgements
G.C.G. and M.T.L. are supported by grants from the National Institutes of Health and the Oak Foundation. S.W. is supported by the Swiss Federal Institute of Technology Zürich (ETHZ), the Swiss National Science Foundation (FNSNF), the European Union and Oncosuisse. Y.B. is supported by the École Polytechnique Fédérale de Lausanne (EPFL), the Centre Hospitalier Universitaire Vaudois Lausanne (CHUV) and the European Consortium for Stem Cell Research (EuroStemCell).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Correspondence should be addressed to G.C.G. or M.T.L. (ggurtner@stanford.edu; longaker@stanford.edu).
Rights and permissions
About this article
Cite this article
Gurtner, G., Werner, S., Barrandon, Y. et al. Wound repair and regeneration. Nature 453, 314–321 (2008). https://doi.org/10.1038/nature07039
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07039
This article is cited by
-
Lonicerin promotes wound healing in diabetic rats by enhancing blood vessel regeneration through Sirt1-mediated autophagy
Acta Pharmacologica Sinica (2024)
-
Activated hepatic stellate cell-derived Bmp-1 induces liver fibrosis via mediating hepatocyte epithelial-mesenchymal transition
Cell Death & Disease (2024)
-
LNP-RNA-engineered adipose stem cells for accelerated diabetic wound healing
Nature Communications (2024)
-
Local and systemic mechanisms that control the hair follicle stem cell niche
Nature Reviews Molecular Cell Biology (2024)
-
Modelling and targeting mechanical forces in organ fibrosis
Nature Reviews Bioengineering (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.