Abstract
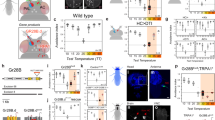
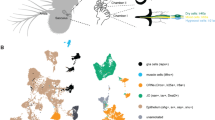
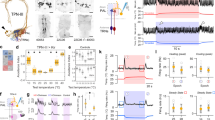
Animals from flies to humans are able to distinguish subtle gradations in temperature and show strong temperature preferences1,2,3,4. Animals move to environments of optimal temperature and some manipulate the temperature of their surroundings, as humans do using clothing and shelter. Despite the ubiquitous influence of environmental temperature on animal behaviour, the neural circuits and strategies through which animals select a preferred temperature remain largely unknown. Here we identify a small set of warmth-activated anterior cell (AC) neurons located in the Drosophila brain, the function of which is critical for preferred temperature selection. AC neuron activation occurs just above the fly’s preferred temperature and depends on dTrpA1, an ion channel that functions as a molecular sensor of warmth. Flies that selectively express dTrpA1 in the AC neurons select normal temperatures, whereas flies in which dTrpA1 function is reduced or eliminated choose warmer temperatures. This internal warmth-sensing pathway promotes avoidance of slightly elevated temperatures and acts together with a distinct pathway for cold avoidance to set the fly’s preferred temperature. Thus, flies select a preferred temperature by using a thermal sensing pathway tuned to trigger avoidance of temperatures that deviate even slightly from the preferred temperature. This provides a potentially general strategy for robustly selecting a narrow temperature range optimal for survival.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fraenkel, G. & Gunn, D. The Orientation of Animals. Kineses, Taxes and Compass Relations (Clarendon, Oxford, 1940)
Fanger, P. O., Ostberg, O., Nicholl, A., Breum, N. O. & Jerking, E. Thermal comfort conditions during day and night. Eur. J. Appl. Physiol. 33, 255–263 (1974)
Sayeed, O. & Benzer, S. Behavioral genetics of thermosensation and hygrosensation in Drosophila. Proc. Natl Acad. Sci. USA 93, 6079–6084 (1996)
Mori, I. Genetics of chemotaxis and thermotaxis in the nematode Caenorhabditis elegans. Annu. Rev. Genet. 33, 399–422 (1999)
Dhaka, A., Viswanath, V. & Patapoutian, A. TRP ion channels and temperature sensation. Annu. Rev. Neurosci. 29, 135–161 (2006)
Rosenzweig, M. et al. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Genes Dev. 19, 419–424 (2005)
Viswanath, V. et al. Opposite thermosensor in fruitfly and mouse. Nature 423, 822–823 (2003)
Siddiqui, W. H. & Barlow, C. A. Population growth of Drosophila melanogaster (Dipetera:Drosophilidae) at constant and alternating temperatures. Ann. Entomol. Soc. Am. 65, 993–1001 (1972)
Nakai, J., Ohkura, M. & Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nature Biotechnol. 19, 137–141 (2001)
Fischer, H. & Tichy, H. Cold-receptor cells supply both cold- and warm-responsive projection neurons in the antennal lobe of the cockroach. J. Comp. Physiol. A Neuroethol. Sens. Neural. Behav. Physiol. 188, 643–648 (2002)
Vosshall, L. B. & Stocker, R. F. Molecular architecture of smell and taste in Drosophila. Annu. Rev. Neurosci. 30, 505–533 (2007)
Ito, K. et al. The organization of extrinsic neurons and their implications in the functional roles of the mushroom bodies in Drosophila melanogaster Meigen. Learn. Mem. 5, 52–77 (1998)
Kitamoto, T., Ikeda, K. & Salvaterra, P. M. Regulation of choline acetyltransferase/lacZ fusion gene expression in putative cholinergic neurons of Drosophila melanogaster. J. Neurobiol. 28, 70–81 (1995)
Brown, A. W. A. Factors in the attractives of bodies for mosquitoes. Nature 167, 202 (1951)
Friend, W. G. & Smith, J. J. B. Factors affecting feeding by bloodsucking insects. Annu. Rev. Entomol. 22, 309–331 (1977)
Parmesan, C. & Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42 (2003)
Patapoutian, A., Peier, A. M., Story, G. M. & Viswanath, V. ThermoTRP channels and beyond: mechanisms of temperature sensation. Nature Rev. Neurosci. 4, 529–539 (2003)
Tominaga, M. & Caterina, M. J. Thermosensation and pain. J. Neurobiol. 61, 3–12 (2004)
Tichy, H. & Gingl, E. Problems in hygro- and thermoreception. In The Ecology of Sensing (eds Barth, F. G. & Schimd, A.) 271–287 (Springer, New York, 2001)
Liu, L., Yermolaieva, O., Johnson, W. A., Abboud, F. M. & Welsh, M. J. Identification and function of thermosensory neurons in Drosophila larvae. Nature Neurosci. 6, 267–273 (2003)
Stevenson, R. D. Body size and limits to the daily range of body temperature in terrestrial ectotherms. Am. Nat. 125, 102–117 (1985)
Heinrich, B. The Hot-Blooded Insects: Strategies and Mechanisms of Thermoregulation (Harvard Univ. Press, Cambridge, USA, 1993)
Caterina, M. J. Transient receptor potential ion channels as participants in thermosensation and thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R64–R76 (2007)
Barolo, S., Castro, B. & Posakony, J. W. New Drosophila transgenic reporters: insulated P-element vectors expressing fast-maturing RFP. Biotechniques 36, 436–440,–442 (2004)
Rong, Y. S. & Golic, K. G. Gene targeting by homologous recombination in Drosophila. Science 288, 2013–2018 (2000)
Kalidas, S. & Smith, D. P. Novel genomic cDNA hybrids produce effective RNA interference in adult Drosophila. Neuron 33, 177–184 (2002)
Tayler, T. D., Robichaux, M. B. & Garrity, P. A. Compartmentalization of visual centers in the Drosophila brain requires Slit and Robo proteins. Development 131, 5935–5945 (2004)
Ng, M. et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron 36, 463–474 (2002)
Acknowledgements
We thank Garrity laboratory members and L. Griffith, L. Huang, R. Huey, E. Marder, C. Miller, M. Rosbash, P. Sengupta, G. Turrigiano and their laboratories for advice and manuscript comments. Supported by NINDS (PO1 NS044232, P30 NS045713S10 and RR16780), NEI (RO1 EY13874, P.A.G.), NIMH (RO1 MH067284, to L. Griffith (for S.P. and A.G.)), and Japan Society for the Promotion of Science (F.N.H.)
Author Contributions F.N.H., M.R., S.R.P., K.K. and P.A.G. designed experiments; F.N.H. performed behaviour and imaging; M.R. created the dTrpA1 mutant, Gal4, RNAi and rescue strains; K.K. performed oocyte electrophysiology and dTrpA1 overexpression; S.R.P. performed NMJ electrophysiology, A.G. assisted with imaging; T.J.J. isolated agTrpA1 cDNA; and P.A.G. performed bioinformatics and assisted with knockdown studies. F.N.H. and P.A.G. wrote the paper with assistance from M.R., K.K., S.R.P. and A.G.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Figures
The file contains Supplementary Figures 1-7 with Legends. (PDF 6902 kb)
Supplementary Movie
The file contains Supplementary Movie 1. Flies that express dTRPA1 in all neurons (c155-Gal4;UAS-dTRPA1; right-hand vial) were temporarily incapacitated by heating to 35°C for 60 seconds, while control flies (which contain only the c155-Gal4 source or the UAS-dTRPA1 transgene alone; left-hand and middle vials, respectively) were not. All flies were heated by submerging their vials in a 35°C water bath for 60 seconds. Heat treatment was done off camera. Some empty frames recorded during heat treatment were removed. The movie is accelerated 8-fold. (MOV 2595 kb)
Rights and permissions
About this article
Cite this article
Hamada, F., Rosenzweig, M., Kang, K. et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature 454, 217–220 (2008). https://doi.org/10.1038/nature07001
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07001
This article is cited by
-
Molecular architecture and gating mechanisms of the Drosophila TRPA1 channel
Cell Discovery (2023)
-
Different spectral sensitivities of ON- and OFF-motion pathways enhance the detection of approaching color objects in Drosophila
Nature Communications (2023)
-
A rapid and bidirectional reporter of neural activity reveals neural correlates of social behaviors in Drosophila
Nature Neuroscience (2023)
-
A Neural Circuit Controlling Virgin Female Aggression Induced by Mating-related Cues in Drosophila
Neuroscience Bulletin (2023)
-
Integration of photoperiodic and temperature cues by the circadian clock to regulate insect seasonal adaptations
Journal of Comparative Physiology A (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.