Abstract
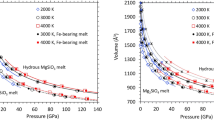
The structure and physical properties of hydrous silicate melts and the solubility of water in melts over most of the pressure regime of Earth’s mantle (up to 136 GPa) remain unknown. At low pressure (up to a few gigapascals) the solubility of water increases rapidly with increasing pressure1, and water has a large influence on the solidus temperature, density2, viscosity3 and electrical conductivity. Here we report the results of first-principles molecular dynamics simulations of hydrous MgSiO3 melt. These show that pressure has a profound influence on speciation of the water component, which changes from being dominated by hydroxyls and water molecules at low pressure4 to extended structures at high pressure. We link this change in structure to our finding that the water–silicate system becomes increasingly ideal at high pressure: we find complete miscibility of water and silicate melt throughout almost the entire mantle pressure regime. On the basis of our results, we argue that a buoyantly stable melt at the base of the upper mantle would contain approximately 3 wt% water and have an electrical conductivity of 18 S m-1, and should therefore be detectable by means of electromagnetic sounding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Shen, A. H. & Keppler, H. Direct observation of complete miscibility in the albite-H2O system. Nature 385, 710–712 (1997)
Ochs, F. A. & Lange, R. A. The density of hydrous magmatic liquids. Science 283, 1314–1317 (1999)
Lange, R. A. The effect of H2O, CO2 and F on the density and viscosity of silicate melts. Rev. Mineral. 30, 331–369 (1994)
Stolper, E. The speciation of water in silicate melts. Geochim. Cosmochim. Acta 46, 2609–2620 (1982)
Panero, W. R. & Stixrude, L. P. Hydrogen incorporation in stishovite at high pressure and symmetric hydrogen bonding in delta-AlOOH. Earth Planet. Sci. Lett. 221, 421–431 (2004)
Pohlmann, M., Benoit, M. & Kob, W. First-principles molecular-dynamics simulations of a hydrous silica melt: Structural properties and hydrogen diffusion mechanism. Phys. Rev. B 70, 184209 (2004)
Stixrude, L. & Karki, B. Structure and freezing of MgSiO3 liquid in Earth’s lower mantle. Science 310, 297–299 (2005)
Closmann, C. & Williams, Q. In-situ spectroscopic investigation of high-pressure hydrated (Mg,Fe)SiO3 glasses - OH vibrations as a probe of glass structure. Am. Mineral. 80, 201–212 (1995)
Inoue, T. Effect of water on melting phase relations and melt composition in the system Mg2SiO4-MgSiO3-H2O up to 15 GPa. Phys. Earth Planet. Inter. 85, 237–263 (1994)
Goldman, N., Fried, L. E., Kuo, I. F. W. & Mundy, C. J. Bonding in the superionic phase of water. Phys. Rev. Lett. 94, 217801 (2005)
Greaves, G. N. & Ngai, K. L. Reconciling ionic-transport properties with atomic-structure in oxide glasses. Phys. Rev. B 52, 6358–6380 (1995)
Song, T. R. A., Helmberger, D. V. & Grand, S. P. Low-velocity zone atop the 410-km seismic discontinuity in the northwestern United States. Nature 427, 530–533 (2004)
Toffelmier, D. A. & Tyburczy, J. A. Electromagnetic detection of a 410-km-deep melt layer in the southwestern United States. Nature 447, 991–994 (2007)
Dziewonski, A. M. & Anderson, D. L. Preliminary reference earth model. Phys. Earth Planet. Inter. 25, 297–356 (1981)
Ito, E. & Takahashi, E. Melting of peridotite at uppermost lower-mantle conditions. Nature 328, 514–517 (1987)
Matsukage, K. N., Jing, Z. C. & Karato, S. Density of hydrous silicate melt at the conditions of Earth's deep upper mantle. Nature 438, 488–491 (2005)
Sakamaki, T., Suzuki, A. & Ohtani, E. Stability of hydrous melt at the base of the Earth's upper mantle. Nature 439, 192–194 (2006)
Stalder, R., Ulmer, P., Thompson, A. B. & Gunther, D. High pressure fluids in the system MgO-SiO2-H2O under upper mantle conditions. Contrib. Mineral. Petrol. 140, 607–618 (2001)
Kerridge, J. F. Carbon, hydrogen and nitrogen in carbonaceous chondrites - abundances and isotopic compositions in bulk samples. Geochim. Cosmochim. Acta 49, 1707–1714 (1985)
Hirschmann, M. M. Water, melting, and the deep Earth H2O cycle. Annu. Rev. Earth Planet. Sci. 34, 629–653 (2006)
Matsui, T. & Abe, Y. Evolution of an impact-induced atmosphere and magma ocean on the accreting Earth. Nature 319, 303–305 (1986)
Nosé, S. A unified formulation of the constant temperature molecular-dynamics methods. J. Chem. Phys. 81, 511–519 (1984)
Kresse, G. & Hafner, J. Norm-conserving and ultrasoft pseudopotentials for first-row and transition-elements. J. Phys. Condens. Matter 6, 8245–8257 (1994)
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996)
Mermin, N. D. Thermal properties of inhomogeneous electron gas. Phys. Rev. 137, A1441–A1443 (1965)
Wentzcovitch, R. M., Martins, J. L. & Allen, P. B. Energy versus free-energy conservation in 1st-principles molecular-dynamics. Phys. Rev. B 45, 11372–11374 (1992)
Zhang, Y. X. H2O in rhyolitic glasses and melts: Measurement, speciation, solubility, and diffusion. Rev. Geophys. 37, 493–516 (1999)
Bhattarai, D., Karki, B. B. & Stixrude, L. Space-time multiresolution atomistic visualization of MgO and MgSiO3 liquid data. Vis. Geosci. 11, 1–11 (2006)
Zhang, Y. X. & Stolper, E. M. Water diffusion in a basaltic melt. Nature 351, 306–309 (1991)
Pitzer, K. S. & Sterner, S. M. Equations of state valid continuously from zero to extreme pressures for H2O and CO2 . J. Chem. Phys. 101, 3111–3116 (1994)
Acknowledgements
Authors thank the Center for Computation & Technology at Louisiana State University for computing resources. This work was supported by the US National Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information
This file contains Supplementary Figure S1a-d plus legend. (PDF 4053 kb)
Rights and permissions
About this article
Cite this article
Mookherjee, M., Stixrude, L. & Karki, B. Hydrous silicate melt at high pressure. Nature 452, 983–986 (2008). https://doi.org/10.1038/nature06918
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06918
This article is cited by
-
Damage mechanism of granite under subcritical water–rock interaction
Environmental Earth Sciences (2023)
-
Hot dense silica glass with ultrahigh elastic moduli
Scientific Reports (2022)
-
Miscibility of rock and ice in the interiors of water worlds
Scientific Reports (2022)
-
Superionic iron alloys and their seismic velocities in Earth’s inner core
Nature (2022)
-
Preparation of Al2O3–Cr2O3 Solid Solutions as Buoyancy Markers and Their High Pressure Synchrotron X-ray Diffraction Analysis
Pure and Applied Geophysics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.