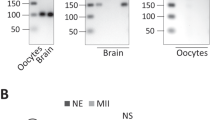
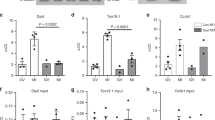
Abstract
In vertebrate oocytes, meiotic progression is driven by the sequential translational activation of maternal messenger RNAs stored in the cytoplasm. This activation is mainly induced by the cytoplasmic elongation of their poly(A) tails, which is mediated by the cytoplasmic polyadenylation element (CPE) present in their 3′ untranslated regions1,2. In Xenopus oocytes, sequential phase-specific translation of CPE-regulated mRNAs is required to activate the maturation-promoting factor, which in turn mediates entry into the two consecutive meiotic metaphases (MI and MII)3,4,5,6. Here we report a genome-wide functional screening to identify previously unknown mRNAs cytoplasmically polyadenylated at meiotic phase transitions. A significant fraction of transcripts containing, in addition to CPEs, (A + U)-rich element (ARE) sequences (characteristic of mRNAs regulated by deadenylation7) were identified. Among these is the mRNA encoding C3H-4, an ARE-binding protein that we find to accumulate in MI and the ablation of which induces meiotic arrest. Our results suggest that C3H-4 recruits the CCR4 deadenylase complex to ARE-containing mRNAs and this, in turn, causes shortening of poly(A) tails. We also show that the opposing activities of the CPEs and the AREs define the precise activation times of the mRNAs encoding the anaphase-promoting complex inhibitors Emi1 and Emi2 during distinct phases of the meiotic cycle. Taken together, our results show that an ‘early’ wave of cytoplasmic polyadenylation activates a negative feedback loop by activating the synthesis of C3H-4, which in turn would recruit the deadenylase complex to mRNAs containing both CPEs and AREs. This negative feedback loop is required to exit from metaphase into interkinesis and for meiotic progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Mendez, R. & Richter, J. D. Translational control by CPEB: a means to the end. Nature Rev. Mol. Cell Biol. 2, 521–529 (2001)
Richter, J. D. CPEB: a life in translation. Trends Biochem. Sci. 32, 279–285 (2007)
de Moor, C. H. & Richter, J. D. The Mos pathway regulates cytoplasmic polyadenylation in Xenopus oocytes. Mol. Cell. Biol. 17, 6419–6426 (1997)
Ballantyne, S., Daniel, D. L. & Wickens, M. A dependent pathway of cytoplasmic polyadenylation reactions linked to cell cycle control by c-mos and CDK1 activation. Mol. Biol. Cell 8, 1633–1648 (1997)
Mendez, R. et al. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature 404, 302–307 (2000)
Mendez, R., Barnard, D. & Richter, J. D. Differential mRNA translation and meiotic progression require Cdc2-mediated CPEB destruction. EMBO J. 21, 1833–1844 (2002)
Voeltz, G. K. & Steitz, J. A. AUUUA sequences direct mRNA deadenylation uncoupled from decay during Xenopus early development. Mol. Cell. Biol. 18, 7537–7545 (1998)
Piqué, M., López, J. M., Foissac, S., Guigó, R. & Méndez, R. A combinatorial code for CPE-mediated translational control. Cell 132, 434–448 (2008)
Mendez, R., Murthy, K. G., Ryan, K., Manley, J. L. & Richter, J. D. Phosphorylation of CPEB by Eg2 mediates the recruitment of CPSF into an active cytoplasmic polyadenylation complex. Mol. Cell 6, 1253–1259 (2000)
Rempel, R. E., Sleight, S. B. & Maller, J. L. Maternal Xenopus Cdk2–cyclin E complexes function during meiotic and early embryonic cell cycles that lack a G1 phase. J. Biol. Chem. 270, 6843–6855 (1995)
Charlesworth, A., Welk, J. & MacNicol, A. M. The temporal control of Wee1 mRNA translation during Xenopus oocyte maturation is regulated by cytoplasmic polyadenylation elements within the 3′-untranslated region. Dev. Biol. 227, 706–719 (2000)
Simon, R. & Richter, J. D. Further analysis of cytoplasmic polyadenylation in Xenopus embryos and identification of embryonic cytoplasmic polyadenylation element-binding proteins. Mol. Cell. Biol. 14, 7867–7875 (1994)
Wu, L., Good, P. J. & Richter, J. D. The 36-kilodalton embryonic-type cytoplasmic polyadenylation element-binding protein in Xenopus laevis is ElrA, a member of the ELAV family of RNA-binding proteins. Mol. Cell. Biol. 17, 6402–6409 (1997)
De, J. et al. Identification of four CCCH zinc finger proteins in Xenopus, including a novel vertebrate protein with four zinc fingers and severely restricted expression. Gene 228, 133–145 (1999)
Lai, W. S., Carballo, E., Thorn, J. M., Kennington, E. A. & Blackshear, P. J. Interactions of CCCH zinc finger proteins with mRNA. Binding of tristetraprolin-related zinc finger proteins to AU-rich elements and destabilization of mRNA. J. Biol. Chem. 275, 17827–17837 (2000)
Parry, D. H., Hickson, G. R. & O’Farrell, P. H. Cyclin B destruction triggers changes in kinetochore behavior essential for successful anaphase. Curr. Biol. 13, 647–653 (2003)
Yang, Z. et al. Silencing mitosin induces misaligned chromosomes, premature chromosome decondensation before anaphase onset, and mitotic cell death. Mol. Cell. Biol. 25, 4062–4074 (2005)
Collart, M. A. Global control of gene expression in yeast by the Ccr4–Not complex. Gene 313, 1–16 (2003)
Morita, M. et al. Depletion of mammalian CCR4b deadenylase triggers increment of the p27Kip1 mRNA level and impairs cell growth. Mol. Cell. Biol. 13, 4980–4990 (2007)
Reimann, J. D. et al. Emi1 is a mitotic regulator that interacts with Cdc20 and inhibits the anaphase promoting complex. Cell 105, 645–655 (2001)
Margottin-Goguet, F. et al. Prophase destruction of Emi1 by the SCF(βTrCP/Slimb) ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell 4, 813–826 (2003)
Tung, J. J. et al. A role for the anaphase-promoting complex inhibitor Emi2/XErp1, a homolog of early mitotic inhibitor 1, in cytostatic factor arrest of Xenopus eggs. Proc. Natl Acad. Sci. USA 102, 4318–4323 (2005)
Ohe, M., Inoue, D., Kanemori, Y. & Sagata, N. Erp1/Emi2 is essential for the meiosis I to meiosis II transition in Xenopus oocytes. Dev. Biol. 303, 157–164 (2007)
Tung, J. J., Padmanabhan, K., Hansen, D. V., Richter, J. D. & Jackson, P. K. Translational unmasking of Emi2 directs cytostatic factor arrest in meiosis II. Cell Cycle 6, 725–731 (2007)
Inoue, D., Ohe, M., Kanemori, Y., Nobui, T. & Sagata, N. A direct link of the Mos–MAPK pathway to Erp1/Emi2 in meiotic arrest of Xenopus laevis eggs. Nature 446, 1100–1104 (2007)
Nishiyama, T., Ohsumi, K. & Kishimoto, T. Phosphorylation of Erp1 by p90rsk is required for cytostatic factor arrest in Xenopus laevis eggs. Nature 446, 1096–1099 (2007)
Liu, J., Grimison, B. & Maller, J. L. New insight into metaphase arrest by cytostatic factor: from establishment to release. Oncogene 26, 1286–1289 (2007)
Ferrell, J. E. Building a cellular switch: more lessons from a good egg. Bioessays 21, 866–870 (1999)
Ferrell, J. E. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14, 140–148 (2002)
Brandman, O., Ferrell, J. E., Li, R. & Meyer, T. Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science 310, 496–498 (2005)
Castro, A., Mandart, E., Lorca, T. & Galas, S. Involvement of Aurora A kinase during meiosis I–II transition in Xenopus oocytes. J. Biol. Chem. 278, 2236–2241 (2003)
Charlesworth, A., Cox, L. L. & MacNicol, A. M. Cytoplasmic polyadenylation element (CPE)- and CPE-binding protein (CPEB)-independent mechanisms regulate early class maternal mRNA translational activation in Xenopus oocytes. J. Biol. Chem. 279, 17650–17659 (2004)
Hake, L. E. & Richter, J. D. CPEB is a specificity factor that mediates cytoplasmic polyadenylation during Xenopus oocyte maturation. Cell 79, 617–627 (1994)
Aoki, K., Matsumoto, K. & Tsujimoto, M. Xenopus cold-inducible RNA-binding protein 2 interacts with ElrA, the Xenopus homolog of HuR, and inhibits deadenylation of specific mRNAs. J. Biol. Chem. 278, 48491–48497 (2003)
Acknowledgements
We thank J. D. Richter for the anti-CPEB antibody; E. Wahle for the dCCR4 antibody; T. U. Mayer for the Emi2 antibody; and M. Fernández, members of the Méndez laboratory, J. Valcarcel and other colleagues from the Gene Expression Program for advice and for critically reading the manuscript. This work was supported by grants from the MEC, Fundación ‘La Caixa’ and Fundació ‘Marató de TV3’. R.M. is a recipient of a contract from the ‘Programa Ramon y Cajal’ (MEC).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
The file contains Supplementary Figures 1-8 with Legends, Supplementary Tables 1-2 and additional references. (PDF 2079 kb)
Rights and permissions
About this article
Cite this article
Belloc, E., Méndez, R. A deadenylation negative feedback mechanism governs meiotic metaphase arrest. Nature 452, 1017–1021 (2008). https://doi.org/10.1038/nature06809
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06809
This article is cited by
-
CPEB and translational control by cytoplasmic polyadenylation: impact on synaptic plasticity, learning, and memory
Molecular Psychiatry (2023)
-
Comparative analyses of vertebrate CPEB proteins define two subfamilies with coordinated yet distinct functions in post-transcriptional gene regulation
Genome Biology (2022)
-
CPEB3-mediated MTDH mRNA translational suppression restrains hepatocellular carcinoma progression
Cell Death & Disease (2020)
-
Noncanonical translation via deadenylated 3′ UTRs maintains primordial germ cells
Nature Chemical Biology (2018)
-
CNOT6 regulates a novel pattern of mRNA deadenylation during oocyte meiotic maturation
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.