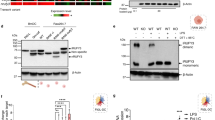
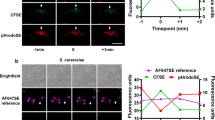
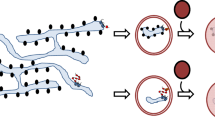
Abstract
Phagocytosis and autophagy are two ancient, highly conserved processes involved, respectively, in the removal of extracellular organisms and the destruction of organisms in the cytosol1,2,3. Autophagy, for either metabolic regulation or defence, involves the formation of a double membrane called the autophagosome, which then fuses with lysosomes to degrade the contents4, a process that has similarities with phagosome maturation. Toll-like-receptor (TLR) engagement activates a variety of defence mechanisms within phagocytes5, including facilitation of phagosome maturation6, and also engages autophagy7. Therefore we speculated that TLR signalling might link these processes to enhance the function of conventional phagosomes. Here we show that a particle that engages TLRs on a murine macrophage while it is phagocytosed triggers the autophagosome marker LC3 to be rapidly recruited to the phagosome in a manner that depends on the autophagy pathway proteins ATG5 and ATG7; this process is preceded by recruitment of beclin 1 and phosphoinositide-3-OH kinase activity. Translocation of beclin 1 and LC3 to the phagosome was not associated with observable double-membrane structures characteristic of conventional autophagosomes, but was associated with phagosome fusion with lysosomes, leading to rapid acidification and enhanced killing of the ingested organism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Levine, B. Eating oneself and uninvited guests: autophagy-related pathways in cellular defense. Cell 120, 159–162 (2005)
Nakagawa, I. et al. Autophagy defends cells against invading group A Streptococcus. Science 306, 1037–1040 (2004)
Singh, S. B., Davis, A. S., Taylor, G. A. & Deretic, V. Human IRGM induces autophagy to eliminate intracellular mycobacteria. Science 313, 1438–1441 (2006)
Levine, B. & Deretic, V. Unveiling the roles of autophagy in innate and adaptive immunity. Nature Rev. Immunol. 7, 767–777 (2007)
Takeuchi, O. & Akira, S. Signaling pathways activated by microorganisms. Curr. Opin. Cell Biol. 19, 185–191 (2007)
Blander, J. M. & Medzhitov, R. Regulation of phagosome maturation by signals from toll-like receptors. Science 304, 1014–1018 (2004)
Xu, Y. et al. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 27, 135–144 (2007)
Tanida, I., Ueno, T. & Kominami, E. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 36, 2503–2518 (2004)
Mizushima, N., Yamamoto, A., Matsui, M., Yoshimori, T. & Ohsumi, Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol. Biol. Cell 15, 1101–1111 (2004)
Ozinsky, A. et al. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl Acad. Sci. USA 97, 13766–13771 (2000)
Wooten, R. M. et al. Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J. Immunol. 168, 348–355 (2002)
Brown, G. D. et al. Dectin-1 is a major beta-glucan receptor on macrophages. J. Exp. Med. 196, 407–412 (2002)
Schmid, D., Pypaert, M. & Munz, C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 26, 79–92 (2007)
Dunn, W. A. Studies on the mechanisms of autophagy: formation of the autophagic vacuole. J. Cell Biol. 110, 1923–1933 (1990)
Ishihara, N. et al. Autophagosome requires specific early Sec proteins for its formation and NSF/SNARE for vacuolar fusion. Mol. Biol. Cell 12, 3690–3702 (2001)
Bernales, S., Schuck, S. & Walter, P. ER-Phagy: selective autophagy of the endoplasmic reticulum. Autophagy 3, 285–287 (2007)
Yorimitsu, T. & Klionsky, D. J. Autophagy: molecular machinery for self-eating. Cell Death Differ. 12 (suppl. 2). 1542–1552 (2005)
Kuo, C. C., Lin, W. T., Liang, C. M. & Liang, S. M. Class I and III phosphatidylinositol 3′-kinase play distinct roles in TLR signaling pathway. J. Immunol. 176, 5943–5949 (2006)
Gillooly, D. J., Simonsen, A. & Stenmark, H. Phosphoinositides and phagocytosis. J. Cell Biol. 155, 15–17 (2001)
Leverrier, Y. et al. Class I phosphoinositide 3-kinase p110β is required for apoptotic cell and Fcγ receptor-mediated phagocytosis by macrophages. J. Biol. Chem. 278, 38437–38442 (2003)
Kanai, F. et al. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nature Cell Biol. 3, 675–678 (2001)
Janssens, S. & Beyaert, R. A universal role for MyD88 in TLR/IL-1R-mediated signaling. Trends Biochem. Sci. 27, 474–482 (2002)
Vasselon, T., Hanlon, W. A., Wright, S. D. & Detmers, P. A. Toll-like receptor 2 (TLR2) mediates activation of stress-activated MAP kinase p38. J. Leukoc. Biol. 71, 503–510 (2002)
Andrade, R. M., Wessendarp, M., Gubbels, M. J., Striepen, B. & Subauste, C. S. CD40 induces macrophage anti-Toxoplasma gondii activity by triggering autophagy-dependent fusion of pathogen-containing vacuoles and lysosomes. J. Clin. Invest. 116, 2366–2377 (2006)
Ogawa, M. et al. Escape of intracellular Shigella from autophagy. Science 307, 727–731 (2005)
Amer, A. O. & Swanson, M. S. Autophagy is an immediate macrophage response to Legionella pneumophila. Cell. Microbiol. 7, 765–778 (2005)
Tilney, L. G., Harb, O. S., Connelly, P. S., Robinson, C. G. & Roy, C. R. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J. Cell Sci. 114, 4637–4650 (2001)
Blander, J. M. & Medzhitov, R. Reply to “Toll-like receptors and phagosome maturation”. Nature Immunol. 8 217–218 doi: 10.1038/ni0307-217b (2007)
Yates, R. M. & Russell, D. G. Phagosome maturation proceeds independently of stimulation of toll-like receptors 2 and 4. Immunity 23, 409–417 (2005)
Russell, D. G. & Yates, R. M. Toll-like receptors and phagosome maturation. Nature Immunol. 8 217 doi: 10.1038/ni0307-217a (2007)
Acknowledgements
We thank P. Murray, M. del Mar Lozano-Cedeño, C.-S. Li, M. Smeltzer, M. Bix and D. Vignali for discussions, and the St Jude Flow Cytometry and Scientific Imaging shared resource facilities for technical assistance. M. Colonna and H. Haecker provided us with essential reagents. This work is supported by grants from the US National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Methods and Supplementary Figures S1-S8 with Legends. (PDF 5160 kb)
Supplementary Movie 1
This file contains Supplementary Movie 1 showing RAW cells expressing GFP-LC3 were fed with uncolored E. coli and confocal images were taken every minute. Representative frames of this movie are shown in Figure S3. (MOV 12912 kb)
Supplementary Movie 2
This file contains Supplementary Movie 2 showing GFP-LC3 in RAW cells translocates to phagosomes containing zymosan (red). Representative frames of this movie are shown in Figure 1c. (MOV 11335 kb)
Supplementary Movie 3
This file contains Supplementary Movie 3 showing RAW cells expressing GFP-LC3 were treated with chloroquine for 5 hours. Endoplasmatic reticulum was then stained with ER-Tracker (red). This movie depicts a full z-stack analysis of the macrophage shown in Figure S4b (MOV 7369 kb)
Supplementary Movie 4
This file contains Supplementary Movie 4 showing RAW cells expressing GFP-LC3 were stained with ER-Tracker (red) and then fed with uncolored zymosan. Note that a small area in the center of the yeast is already red before uptake by the macrophage. A representative frame of this video is shown in Figure S4c. (MOV 50299 kb)
Supplementary Movie 5
This file contains Supplementary Movie 5 showing RAW cells expressing GFP-LC3 and the PX-domain of p40 (phox) fused to mCherry (red) were fed with uncolored zymosan. The internalization of a yeast particle was followed at 1 min intervals. Representative frames of this movie are shown in Figure 3g. (MOV 566 kb)
Supplementary Movie 6
This file contains Supplementary Movie 6 showing GFP-LC3/PX-mCherry expressing macrophages were fed with uncolored zymosan and confocal images were taken every minute. Representative frames of this movie are shown in Figure S7. (MOV 15740 kb)
Supplementary Movie 7
This file contains Supplementary Movie 7 showing GFP-LC3/PX-mCherry expressing macrophages were fed with uncolored beads and confocal images were taken every minute. Representative frames of this movie are shown in Figure S7. (MOV 8104 kb)
Supplementary Movie 8
This file contains Supplementary Movie 8 showing Internalization of zymosan (red) was followed at 1.5 min. intervals in RAW cells transiently transfected with GFP-Beclin-1. Representative images of this video are shown in Figure 3h. (MOV 7792 kb)
Supplementary Movie 9
This file contains Supplementary Movie 9 showing Phagocytosis of beads (uncolored) was followed at 1.5 min. intervals in RAW cells transiently transfected with GFP-Beclin-1. (MOV 15584 kb)
Supplementary Movie 10
This file contains Supplementary Movie 10 showing GFP-LC3 expressing RAW cells were preloaded with lysotracker (red) and the phagocytosis of control beads was followed at 3 min. intervals. Representative frames of this video are shown in Figure 4a. (MOV 18892 kb)
Supplementary Movie 11
This file contains Supplementary Movie 11 showing GFP-LC3 expressing RAW cells were preloaded with lysotracker (red) and the internalization of PAM3CSK4-covered beads was followed. Representative frames from this video are shown in Figure 4a. (MOV 28052 kb)
Rights and permissions
About this article
Cite this article
Sanjuan, M., Dillon, C., Tait, S. et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 450, 1253–1257 (2007). https://doi.org/10.1038/nature06421
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature06421
This article is cited by
-
The involvement of α-synucleinopathy in the disruption of microglial homeostasis contributes to the pathogenesis of Parkinson’s disease
Cell Communication and Signaling (2024)
-
P38-DAPK1 axis regulated LC3-associated phagocytosis (LAP) of microglia in an in vitro subarachnoid hemorrhage model
Cell Communication and Signaling (2023)
-
Autophagy and autophagy-related pathways in cancer
Nature Reviews Molecular Cell Biology (2023)
-
Association of Glial Activation and α-Synuclein Pathology in Parkinson’s Disease
Neuroscience Bulletin (2023)
-
CTpathway: a CrossTalk-based pathway enrichment analysis method for cancer research
Genome Medicine (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.