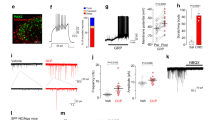
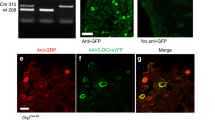
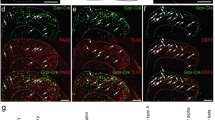
Abstract
Itching, or pruritus, is defined as an unpleasant cutaneous sensation that serves as a physiological self-protective mechanism to prevent the body from being hurt by harmful external agents. Chronic itch represents a significant clinical problem resulting from renal diseases and liver diseases, as well as several serious skin diseases such as atopic dermatitis1,2,3. The identity of the itch-specific mediator in the central nervous system, however, remains elusive. Here we describe that the gastrin-releasing peptide receptor (GRPR) plays an important part in mediating itch sensation in the dorsal spinal cord. We found that gastrin-releasing peptide is specifically expressed in a small subset of peptidergic dorsal root ganglion neurons, whereas expression of its receptor GRPR is restricted to lamina I of the dorsal spinal cord. GRPR mutant mice showed comparable thermal, mechanical, inflammatory and neuropathic pain responses relative to wild-type mice. In contrast, induction of scratching behaviour was significantly reduced in GRPR mutant mice in response to pruritogenic stimuli, whereas normal responses were evoked by painful stimuli. Moreover, direct spinal cerebrospinal fluid injection of a GRPR antagonist significantly inhibited scratching behaviour in three independent itch models. These data demonstrate that GRPR is required for mediating the itch sensation rather than pain, at the spinal level. Our results thus indicate that GRPR may represent the first molecule that is dedicated to mediating the itch sensation in the dorsal horn of the spinal cord, and thus may provide a central therapeutic target for antipruritic drug development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Paus, R., Schmelz, M., Biro, T. & Steinhoff, M. Frontiers in pruritus research: scratching the brain for more effective itch therapy. J. Clin. Invest. 116, 1174–1186 (2006)
Ikoma, A., Steinhoff, M., Stander, S., Yosipovitch, G. & Schmelz, M. The neurobiology of itch. Nature Rev. Neurosci. 7, 535–547 (2006)
Greaves, M. W. Itch in systemic disease: therapeutic options. Dermatol. Ther. 18, 323–327 (2005)
Roesler, R., Henriques, J. A. & Schwartsmann, G. Gastrin-releasing peptide receptor as a molecular target for psychiatric and neurological disorders. CNS Neurol. Disord. Drug Targets 5, 197–204 (2006)
Battey, J. & Wada, E. Two distinct receptor subtypes for mammalian bombesin-like peptides. Trends Neurosci. 14, 524–528 (1991)
Cowan, A., Khunawat, P., Zhu, X. Z. & Gmerek, D. E. Effects of bombesin on behavior. Life Sci. 37, 135–145 (1985)
Gmerek, D. E. & Cowan, A. Studies on bombesin-induced grooming in rats. Peptides 4, 907–913 (1983)
Gmerek, D. E. & Cowan, A. Bombesin—a central mediator of pruritus? Br. J. Dermatol. 109, 239 (1983)
O'Donohue, T. L. et al. A role for bombesin in sensory processing in the spinal cord. J. Neurosci. 4, 2956–2962 (1984)
Moody, T. W. & Merali, Z. Bombesin-like peptides and associated receptors within the brain: distribution and behavioral implications. Peptides 25, 511–520 (2004)
Schmelz, M., Schmidt, R., Bickel, A., Handwerker, H. O. & Torebjork, H. E. Specific C-receptors for itch in human skin. J. Neurosci. 17, 8003–8008 (1997)
Andrew, D. & Craig, A. D. Spinothalamic lamina I neurons selectively sensitive to histamine: a central neural pathway for itch. Nature Neurosci. 4, 72–77 (2001)
Kuraishi, Y., Nagasawa, T., Hayashi, K. & Satoh, M. Scratching behavior induced by pruritogenic but not algesiogenic agents in mice. Eur. J. Pharmacol. 275, 229–233 (1995)
Nystedt, S., Emilsson, K., Wahlestedt, C. & Sundelin, J. Molecular cloning of a potential proteinase activated receptor. Proc. Natl Acad. Sci. USA 91, 9208–9212 (1994)
Steinhoff, M. et al. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nature Med. 6, 151–158 (2000)
Steinhoff, M. et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J. Neurosci. 23, 6176–6180 (2003)
Shimada, S. G., Shimada, K. A. & Collins, J. G. Scratching behavior in mice induced by the proteinase-activated receptor-2 agonist, SLIGRL-NH2. Eur. J. Pharmacol. 530, 281–283 (2006)
Inan, S. & Cowan, A. Kappa opioid agonists suppress chloroquine-induced scratching in mice. Eur. J. Pharmacol. 502, 233–237 (2004)
Green, A. D., Young, K. K., Lehto, S. G., Smith, S. B. & Mogil, J. S. Influence of genotype, dose and sex on pruritogen-induced scratching behavior in the mouse. Pain 124, 50–58 (2006)
Ladenheim, E. E., Taylor, J. E., Coy, D. H., Moore, K. A. & Moran, T. H. Hindbrain GRP receptor blockade antagonizes feeding suppression by peripherally administered GRP. Am. J. Physiol. 271, R180–R184 (1996)
Wang, L. H. et al. des-Met carboxyl-terminally modified analogues of bombesin function as potent bombesin receptor antagonists, partial agonists, or agonists. J. Biol. Chem. 265, 15695–15703 (1990)
Stander, S. & Schmelz, M. Chronic itch and pain—similarities and differences. Eur. J. Pain 10, 473–478 (2006)
McMahon, S. B. & Koltzenburg, M. Itching for an explanation. Trends Neurosci. 15, 497–501 (1992)
Shumyatsky, G. P. et al. Identification of a signaling network in lateral nucleus of amygdala important for inhibiting memory specifically related to learned fear. Cell 111, 905–918 (2002)
Kroog, G. S., Jensen, R. T. & Battey, J. F. Mammalian bombesin receptors. Med. Res. Rev. 15, 389–417 (1995)
Sowunmi, A., Walker, O. & Salako, L. A. Pruritus and antimalarial drugs in Africans. Lancet 2, 213 (1989)
Hampton, L. L. et al. Loss of bombesin-induced feeding suppression in gastrin-releasing peptide receptor-deficient mice. Proc. Natl Acad. Sci. USA 95, 3188–3192 (1998)
Chen, Z. F. et al. The paired homeodomain protein DRG11 is required for the projection of cutaneous sensory afferent fibers to the dorsal spinal cord. Neuron 31, 59–73 (2001)
Wozniak, D. F. et al. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol. Dis. 17, 403–414 (2004)
Hylden, J. L. & Wilcox, G. L. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 67, 313–316 (1980)
Acknowledgements
We thank J. Yin, C.-S. Qiu and K.-H. Zhang for technical assistance and J. Battey for providing GRPR mutant mice. We are grateful to A. Basbaum, E. Carstens, L. Hampton and J. Battey for critical comments on the manuscript. We also thank D. H. Coy for providing the GRPR antagonist. The work was supported by an NIH RO1 to Z.F.C.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Figures 1-7 with Legends and additional references. (PDF 710 kb)
Rights and permissions
About this article
Cite this article
Sun, YG., Chen, ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703 (2007). https://doi.org/10.1038/nature06029
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature06029
This article is cited by
-
Pituitary transcriptome profile from laying period to incubation period of Changshun green-shell laying hens
BMC Genomics (2024)
-
The COL6A5-p.Glu2272* mutation induces chronic itch in mice
Mammalian Genome (2024)
-
Role of GRPR in Acupuncture Intervention in the “Itch-scratch Vicious Cycle” Spinal Circuit of Chronic Pruritus
Chinese Medicine (2023)
-
Characterisation of NPFF-expressing neurons in the superficial dorsal horn of the mouse spinal cord
Scientific Reports (2023)
-
Molecular recognition of itch-associated neuropeptides by bombesin receptors
Cell Research (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.