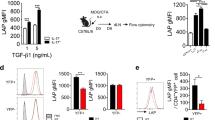
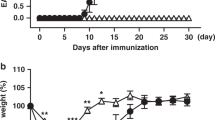
Abstract
On activation, naive T cells differentiate into effector T-cell subsets with specific cytokine phenotypes and specialized effector functions1. Recently a subset of T cells, distinct from T helper (TH)1 and TH2 cells, producing interleukin (IL)-17 (TH17) was defined and seems to have a crucial role in mediating autoimmunity and inducing tissue inflammation2,3,4,5. We and others have shown that transforming growth factor (TGF)-β and IL-6 together induce the differentiation of TH17 cells, in which IL-6 has a pivotal function in dictating whether T cells differentiate into Foxp3+ regulatory T cells (Treg cells) or TH17 cells6,7,8,9. Whereas TGF-β induces Foxp3 and generates Treg cells, IL-6 inhibits the generation of Treg cells and induces the production of IL-17, suggesting a reciprocal developmental pathway for TH17 and Treg cells. Here we show that IL-6-deficient (Il6-/-) mice do not develop a TH17 response and their peripheral repertoire is dominated by Foxp3+ Treg cells. However, deletion of Treg cells leads to the reappearance of TH17 cells in Il6-/- mice, suggesting an additional pathway by which TH17 cells might be generated in vivo. We show that an IL-2 cytokine family member, IL-21, cooperates with TGF-β to induce TH17 cells in naive Il6-/- T cells and that IL-21-receptor-deficient T cells are defective in generating a TH17 response.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Abbas, A. K., Murphy, K. M. & Sher, A. Functional diversity of helper T lymphocytes. Nature 383, 787–793 (1996)
Cua, D. J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003)
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005)
Weaver, C. T., Hatton, R. D., Mangan, P. R. & Harrington, L. E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25, 821–852 (2007)
Steinman, L. A brief history of TH17, the first major revision in the TH1/TH2 hypothesis of T cell-mediated tissue damage. Nature Med. 13, 139–145 (2007)
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006)
Veldhoen, M., Hocking, R. J., Flavell, R. A. & Stockinger, B. Signals mediated by transforming growth factor-β initiate autoimmune encephalomyelitis, but chronic inflammation is needed to sustain disease. Nature Immunol. 7, 1151–1156 (2006)
Mangan, P. R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006)
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006)
Bettelli, E., Oukka, M. & Kuchroo, V. K. TH17 cells in the circle of immunity and autoimmunity. Nature Immunol. 8, 345–350 (2007)
Korn, T. et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nature Med. 13, 423–431 (2007)
Samoilova, E. B., Horton, J. L., Hilliard, B., Liu, T. S. & Chen, Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J. Immunol. 161, 6480–6486 (1998)
Okuda, Y. et al. IL-6-deficient mice are resistant to the induction of experimental autoimmune encephalomyelitis provoked by myelin oligodendrocyte glycoprotein. Int. Immunol. 10, 703–708 (1998)
Mendel, I., Katz, A., Kozak, N., Ben-Nun, A. & Revel, M. Interleukin-6 functions in autoimmune encephalomyelitis: a study in gene-targeted mice. Eur. J. Immunol. 28, 1727–1737 (1998)
Eugster, H. P., Frei, K., Kopf, M., Lassmann, H. & Fontana, A. IL-6-deficient mice resist myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. Eur. J. Immunol. 28, 2178–2187 (1998)
Okuda, Y. et al. IL-6 plays a crucial role in the induction phase of myelin oligodendrocyte glucoprotein 35–55 induced experimental autoimmune encephalomyelitis. J. Neuroimmunol. 101, 188–196 (1999)
Batten, M. et al. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nature Immunol. 7, 929–936 (2006)
Stumhofer, J. S. et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nature Immunol. 7, 937–945 (2006)
Ivanov, I. I. et al. The orphan nuclear receptor RORγt directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell 126, 1121–1133 (2006)
Kasaian, M. T. et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: a mediator of the transition from innate to adaptive immunity. Immunity 16, 559–569 (2002)
Yamanouchi, J. et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nature Genet. 39, 329–337 (2007)
Leonard, W. J. & Spolski, R. Interleukin-21: a modulator of lymphoid proliferation, apoptosis and differentiation. Nature Rev. Immunol. 5, 688–698 (2005)
Zeng, R. et al. The molecular basis of IL-21-mediated proliferation. Blood 109, 4135–4142 (2007)
Yang, X. O. et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282, 9358–9363 (2007)
Murphy, E. et al. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J. Exp. Med. 183, 901–913 (1996)
Nakamura, T., Kamogawa, Y., Bottomly, K. & Flavell, R. A. Polarization of IL-4- and IFN-γ-producing CD4+ T cells following activation of naive CD4+ T cells. J. Immunol. 158, 1085–1094 (1997)
Kleinschek, M. A. et al. IL-25 regulates Th17 function in autoimmune inflammation. J. Exp. Med. 204, 161–170 (2007)
Vollmer, T. L. et al. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. J. Immunol. 174, 2696–2701 (2005)
Reddy, J. et al. Detection of autoreactive myelin proteolipid protein 139–151-specific T cells by using MHC II (IAs) tetramers. J. Immunol. 170, 870–877 (2003)
Bettelli, E. et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081 (2003)
Acknowledgements
We thank M. Collins for providing Il21r-/- mice, and D. Kozoriz, S. Tente, R. Chandwaskar and D. Lee for cell sorting and technical assistance. This work was supported by grants from the National Multiple Sclerosis Society, the National Institutes of Health, the Juvenile Diabetes Research Foundation Center for Immunological Tolerance at Harvard, and the Deutsche Forschungsgemeinschaft. V.K.K. is the recipient of the Javits Neuroscience Investigator Award from the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures S1-S5 with Legends. (PDF 791 kb)
Rights and permissions
About this article
Cite this article
Korn, T., Bettelli, E., Gao, W. et al. IL-21 initiates an alternative pathway to induce proinflammatory TH17 cells. Nature 448, 484–487 (2007). https://doi.org/10.1038/nature05970
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05970
This article is cited by
-
Ceria-vesicle nanohybrid therapeutic for modulation of innate and adaptive immunity in a collagen-induced arthritis model
Nature Nanotechnology (2023)
-
The potential roles of Th17 cells in the pathogenesis of oral lichen planus
Inflammation Research (2023)
-
Three Adult Cases of STAT1 Gain-of-Function with Chronic Mucocutaneous Candidiasis Treated with JAK Inhibitors
Journal of Clinical Immunology (2023)
-
Peptidylarginine deiminase 2 promotes T helper 17-like T cell activation and activated T cell-autonomous death (ACAD) through an endoplasmic reticulum stress and autophagy coupling mechanism
Cellular & Molecular Biology Letters (2022)
-
The role of Th17 cells in the pathogenesis and treatment of breast cancer
Cancer Cell International (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.