Abstract
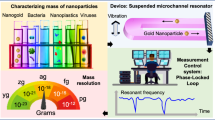
Nanomechanical resonators enable the measurement of mass with extraordinary sensitivity1,2,3,4,5,6,7. Previously, samples as light as 7 zeptograms (1 zg = 10-21 g) have been weighed in vacuum, and proton-level resolution seems to be within reach8. Resolving small mass changes requires the resonator to be light and to ring at a very pure tone—that is, with a high quality factor9. In solution, viscosity severely degrades both of these characteristics, thus preventing many applications in nanotechnology and the life sciences where fluid is required10. Although the resonant structure can be designed to minimize viscous loss, resolution is still substantially degraded when compared to measurements made in air or vacuum11,12,13,14. An entirely different approach eliminates viscous damping by placing the solution inside a hollow resonator that is surrounded by vacuum15,16. Here we demonstrate that suspended microchannel resonators can weigh single nanoparticles, single bacterial cells and sub-monolayers of adsorbed proteins in water with sub-femtogram resolution (1 Hz bandwidth). Central to these results is our observation that viscous loss due to the fluid is negligible compared to the intrinsic damping of our silicon crystal resonator. The combination of the low resonator mass (100 ng) and high quality factor (15,000) enables an improvement in mass resolution of six orders of magnitude over a high-end commercial quartz crystal microbalance17. This gives access to intriguing applications, such as mass-based flow cytometry, the direct detection of pathogens, or the non-optical sizing and mass density measurement of colloidal particles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thundat, T., Wachter, E. A., Sharp, S. L. & Warmack, R. J. Detection of mercury vapor using resonating microcantilevers. Appl. Phys. Lett. 66, 1695–1697 (1995)
Lange, D., Hagleitner, C., Hierlemann, A., Brand, O. & Baltes, H. Complementary metal oxide semiconductor cantilever arrays on a single chip: Mass-sensitive detection of volatile organic compounds. Anal. Chem. 74, 3084–3095 (2002)
Lavrik, N. V. & Datskos, P. G. Femtogram mass detection using photothermally actuated nanomechanical resonators. Appl. Phys. Lett. 82, 2697–2699 (2003)
Ono, T., Li, X., Miyashita, H. & Esashi, M. Mass sensing of adsorbed molecules in sub-picogram sample with ultrathin silicon resonator. Rev. Sci. Instrum. 74, 1240–1243 (2003)
Gupta, A., Akin, D. & Bashir, R. Single virus particle mass detection using microresonators with nanoscale thickness. Appl. Phys. Lett. 84, 1976–1978 (2004)
Ilic, B. et al. Attogram detection using nanoelectromechanical oscillators. J. Appl. Phys. 95, 3694–3703 (2004)
Forsen, E. et al. Ultrasensitive mass sensor fully integrated with complementary metal-oxide-semiconductor circuitry. Appl. Phys. Lett. 87, 043507 (2005)
Yang, Y. T., Callegari, C., Feng, X. L., Ekinci, K. L. & Roukes, M. L. Zeptogram-scale nanomechanical mass sensing. Nano Lett. 6, 583–586 (2006)
Ekinci, K. L., Yang, Y. T. & Roukes, M. L. Ultimate limits to inertial mass sensing based upon nanoelectromechanical systems. J. Appl. Phys. 95, 2682–2689 (2004)
Lucklum, R. & Hauptmann, P. Acoustic microsensors—the challenge behind microgravimetry. Anal. Bioanal. Chem. 384, 667–682 (2006)
Weinberg, M. S., Dube, C. E., Petrovich, A. & Zapata, A. M. Fluid damping in resonant flexural plate wave device. J. Microelectromech. Syst. 12, 567–576 (2003)
Zhang, H. & Kim, E. S. Micromachined acoustic resonant mass sensor. J. Microelectromech. Syst. 14, 699–706 (2005)
Braun, T. et al. Micromechanical mass sensors for biomolecular detection in a physiological environment. Phys. Rev. E 72, 031907 (2005)
Pang, W. et al. Femtogram mass sensing platform based on lateral extensional mode piezoelectric resonator. Appl. Phys. Lett. 88, 243503 (2006)
Burg, T. P. & Manalis, S. R. Suspended microchannel resonators for biomolecular detection. Appl. Phys. Lett. 83, 2698–2700 (2003)
Burg, T. P. et al. Vacuum-packaged suspended microchannel resonant mass sensor for biomolecular detection. J. Microelectromech. Syst. 15, 1466–1476 (2006)
Q-Sense. Model D3000 specifications. 〈http://www.q-sense.com/viewArticle.asp?ID=31〉.
Enoksson, P., Stemme, G. & Stemme, E. Silicon tube structures for a fluid-density sensor. Sens. Actuators A 54, 558–562 (1996)
Westberg, D., Paul, O., Andersson, G. & Baltes, H. A CMOS-compatible fluid density sensor. J. Micromech. Microeng. 7, 253–255 (1997)
Sarid, D. Scanning Force Microscopy: With Applications to Electric, Magnetic, and Atomic Forces (Oxford Univ. Press, USA, 1994)
Myszka, D. G. Analysis of small-molecule interactions using Biacore S51 technology. Anal. Biochem. 329, 316–323 (2004)
Angenendt, P., Glokler, J., Sobek, J., Lehrach, H. & Cahill, D. J. Next generation of protein microarray support materials: Evaluation for protein and antibody microarray applications. J. Chromatogr. A 1009, 97–104 (2003)
Wu, G. H. et al. Bioassay of prostate-specific antigen (PSA) using microcantilevers. Nature Biotechnol. 19, 856–860 (2001)
Backmann, N. et al. A label-free immunosensor array using single-chain antibody fragments. Proc. Natl Acad. Sci. USA 102, 14587–14592 (2005)
Akerlund, T., Nordstrom, K. & Bernander, R. Analysis of cell size and DNA content in exponentially growing and stationary-phase batch cultures of Escherichia coli. J. Bacteriol. 177, 6791–6797 (1995)
Nam, J. M., Thaxton, C. S. & Mirkin, C. A. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 301, 1884–1886 (2003)
Agrawal, A., Zhang, C. Y., Byassee, T., Tripp, R. A. & Nie, S. M. Counting single native biomolecules and intact viruses with color-coded nanoparticles. Anal. Chem. 78, 1061–1070 (2006)
Thorsen, T., Maerkl, S. J. & Quake, S. R. Microfluidic large-scale integration. Science 298, 580–584 (2002)
Whitesides, G. M. The origins and the future of microfluidics. Nature 442, 368–373 (2006)
Yager, P. et al. Microfluidic diagnostic technologies for global public health. Nature 442, 412–418 (2006)
Acknowledgements
We thank N. Milovic, J. Behr, M.T. Thompson and K. Van Vliet for helpful discussions, A. Mirza for substantial contributions to device fabrication, and A. Ting for a critical review of the manuscript. We also acknowledge financial support from the National Institutes of Health (NIH) Cell Decision Process Center Grant, the Institute for Collaborative Biotechnologies from the US Army Research Office, the Air Force Office of Sponsored Research and a National Science Foundation (NSF) Small Business Innovation Research award. M.G. acknowledges support from the Natural Sciences and Engineering Research Council of Canada (NSERC) through a postdoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Competing interests: S.R.M. and K.B. hold equity in, and are co-founders of, Affinity Biosensors, which will develop commercial instruments for applications described in this paper. W.S., G.C. and J.S.F. are employed by Innovative Micro Technology, which manufactures the devices described in this paper as part of a partnership with Affinity Biosensors.
Supplementary information
Supplementary Information
This file contains Supplementary Methods with additional details regarding device fabrication and experimental procedures, and a Supplementary Discussion comparing the suspended microchannel resonator with other mass sensing methods in fluid. The file includes Supplementary Figures S1 and S2, and Supplementary Table S1. (PDF 254 kb)
Rights and permissions
About this article
Cite this article
Burg, T., Godin, M., Knudsen, S. et al. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature 446, 1066–1069 (2007). https://doi.org/10.1038/nature05741
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05741
This article is cited by
-
Leukemia circulation kinetics revealed through blood exchange method
Communications Biology (2024)
-
Quantifying the performances of SU-8 microfluidic devices: high liquid water tightness, long-term stability, and vacuum compatibility
Microfluidics and Nanofluidics (2024)
-
Advanced operation of heated fluidic resonators via mechanical and thermal loss reduction in vacuum
Microsystems & Nanoengineering (2023)
-
Flexibly designable wettability gradient for passive control of fluid motion via physical surface modification
Scientific Reports (2023)
-
A time fractional model of a Maxwell nanofluid through a channel flow with applications in grease
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.