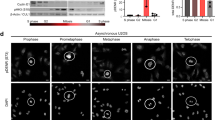
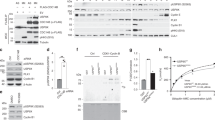
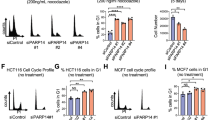
Abstract
14-3-3 proteins are crucial in a wide variety of cellular responses including cell cycle progression, DNA damage checkpoints and apoptosis. One particular 14-3-3 isoform, σ, is a p53-responsive gene, the function of which is frequently lost in human tumours, including breast and prostate cancers as a result of either hypermethylation of the 14-3-3σ promoter or induction of an oestrogen-responsive ubiquitin ligase that specifically targets 14-3-3σ for proteasomal degradation1,2,3,4,5,6,7,8,9. Loss of 14-3-3σ protein occurs not only within the tumours themselves but also in the surrounding pre-dysplastic tissue (so-called field cancerization), indicating that 14-3-3σ might have an important tumour suppressor function that becomes lost early in the process of tumour evolution3,9. The molecular basis for the tumour suppressor function of 14-3-3σ is unknown. Here we report a previously unknown function for 14-3-3σ as a regulator of mitotic translation through its direct mitosis-specific binding to a variety of translation/initiation factors, including eukaryotic initiation factor 4B in a stoichiometric manner. Cells lacking 14-3-3σ, in marked contrast to normal cells, cannot suppress cap-dependent translation and do not stimulate cap-independent translation during and immediately after mitosis. This defective switch in the mechanism of translation results in reduced mitotic-specific expression of the endogenous internal ribosomal entry site (IRES)-dependent form of the cyclin-dependent kinase Cdk11 (p58 PITSLRE), leading to impaired cytokinesis, loss of Polo-like kinase-1 at the midbody, and the accumulation of binucleate cells. The aberrant mitotic phenotype of 14-3-3σ-depleted cells can be rescued by forced expression of p58 PITSLRE or by extinguishing cap-dependent translation and increasing cap-independent translation during mitosis by using rapamycin. Our findings show how aberrant mitotic translation in the absence of 14-3-3σ impairs mitotic exit to generate binucleate cells and provides a potential explanation of how 14-3-3σ-deficient cells may progress on the path to aneuploidy and tumorigenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
11 April 2007
The name Steven A. Artim has been corrected to Stephen C. Artim
References
Vercoutter-Edouart, A. S. et al. Proteomic analysis reveals that 14-3-3σ is down-regulated in human breast cancer cells. Cancer Res. 61, 76–80 (2001)
Ferguson, A. T. et al. High frequency of hypermethylation at the 14-3-3σ locus leads to gene silencing in breast cancer. Proc. Natl Acad. Sci. USA 97, 6049–6054 (2000)
Umbricht, C. B. et al. Hypermethylation of 14-3-3σ (stratifin) is an early event in breast cancer. Oncogene 20, 3348–3353 (2001)
Urano, T. et al. Efp targets 14-3-3σ for proteolysis and promotes breast tumour growth. Nature 417, 871–875 (2002)
Moreira, J. M., Gromov, P. & Celis, J. E. Expression of the tumor suppressor protein 14-3-3σ is down-regulated in invasive transitional cell carcinomas of the urinary bladder undergoing epithelial-to-mesenchymal transition. Mol. Cell. Proteomics 3, 410–419 (2004)
Iwata, N. et al. Frequent hypermethylation of CpG islands and loss of expression of the 14-3-3σ gene in human hepatocellular carcinoma. Oncogene 19, 5298–5302 (2000)
Suzuki, H. et al. Inactivation of the 14-3-3σ gene is associated with 5′ CpG island hypermethylation in human cancers. Cancer Res. 60, 4353–4357 (2000)
Villaret, D. B. et al. Identification of genes overexpressed in head and neck squamous cell carcinoma using a combination of complementary DNA subtraction and microarray analysis. Laryngoscope 110, 374–381 (2000)
Gasco, M. et al. Coincident inactivation of 14-3-3σ and p16INK4a is an early event in vulval squamous neoplasia. Oncogene 21, 1876–1881 (2002)
Prescott, A. R. Synthesis of RNA and protein during mitosis in mammalian tissue culture cells. Exp. Cell Res. 26, 260–268 (1962)
Pyronnet, S., Dostie, J. & Sonenberg, N. Suppression of cap-dependent translation in mitosis. Genes Dev. 15, 2083–2093 (2001)
Pyronnet, S., Pradayrol, L. & Sonenberg, N. A cell cycle-dependent internal ribosome entry site. Mol. Cell 5, 607–616 (2000)
Qin, X. & Sarnow, P. Preferential translation of internal ribosome entry site-containing mRNAs during the mitotic cycle in mammalian cells. J. Biol. Chem. 279, 13721–13728 (2004)
Brasey, A. et al. The leader of human immunodeficiency virus type 1 genomic RNA harbors an internal ribosome entry segment that is active during the G2/M phase of the cell cycle. J. Virol. 77, 3939–3949 (2003)
Kullmann, M., Gopfert, U., Siewe, B. & Hengst, L. B. ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5′UTR. Genes Dev. 16, 3087–3099 (2002)
Hershey, J. W. Expression of initiation factor genes in mammalian cells. Biochimie 76, 847–852 (1994)
Holcik, M. & Sonenberg, N. Translational control in stress and apoptosis. Nature Rev. Mol. Cell Biol. 6, 318–327 (2005)
Merrick, W. C. Cap-dependent and cap-independent translation in eukaryotic systems. Gene 332, 1–11 (2004)
Rogers, G. W., Richter, N. J. & Merrick, W. C. Biochemical and kinetic characterization of the RNA helicase activity of eukaryotic initiation factor 4A. J. Biol. Chem. 274, 12236–12244 (1999)
Neef, R. et al. Phosphorylation of mitotic kinesin-like protein 2 by polo-like kinase 1 is required for cytokinesis. J. Cell Biol. 162, 863–875 (2003)
Liu, X., Zhou, T., Kuriyama, R. & Erikson, R. L. Molecular interactions of Polo-like-kinase 1 with the mitotic kinesin-like protein CHO1/MKLP-1. J. Cell Sci. 117, 3233–3246 (2004)
Beretta, L., Gingras, A. C., Svitkin, Y. V., Hall, M. N. & Sonenberg, N. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 15, 658–664 (1996)
Li, T., Inoue, A., Lahti, J. M. & Kidd, V. J. Failure to proliferate and mitotic arrest of CDK11p110/p58-null mutant mice at the blastocyst stage of embryonic cell development. Mol. Cell. Biol. 24, 3188–3197 (2004)
Petretti, C. et al. The PITSLRE/CDK11p58 protein kinase promotes centrosome maturation and bipolar spindle formation. EMBO Rep. 7, 418–424 (2006)
Cornelis, S. et al. Identification and characterization of a novel cell cycle-regulated internal ribosome entry site. Mol. Cell 5, 597–605 (2000)
Shi, Q. & King, R. W. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature 437, 1038–1042 (2005)
Fujiwara, T. et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437, 1043–1047 (2005)
Brunet, A. et al. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J. Cell Biol. 156, 817–828 (2002)
Wilker, E. W., Grant, R. A., Artim, S. C. & Yaffe, M. B. A structural basis for 14-3-3σ functional specificity. J. Biol. Chem. 280, 18891–18898 (2005)
Rubinson, D. A. et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nature Genet. 33, 401–406 (2003)
Acknowledgements
We thank P. Stern, D. Lowery and W. Merrick for reagents and technical assistance. This work was supported by postdoctoral fellowships from the Anna Fuller Fund and the NIH to E.W.W., an EMBO long-term fellowship to M.A.T.M.v.V., the David H. Koch Cancer Research Fund, NIH grants, and a Burroughs-Wellcome Career Development Award to M.B.Y.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Table 1, Supplementary Figures 1-4 with Legends and additional references. (PDF 3669 kb)
Rights and permissions
About this article
Cite this article
Wilker, E., van Vugt, M., Artim, S. et al. 14-3-3σ controls mitotic translation to facilitate cytokinesis. Nature 446, 329–332 (2007). https://doi.org/10.1038/nature05584
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature05584
This article is cited by
-
Cyclin B/CDK1 and Cyclin A/CDK2 phosphorylate DENR to promote mitotic protein translation and faithful cell division
Nature Communications (2022)
-
Using a machine learning approach to identify key prognostic molecules for esophageal squamous cell carcinoma
BMC Cancer (2021)
-
Secreted midbody remnants are a class of extracellular vesicles molecularly distinct from exosomes and microparticles
Communications Biology (2021)
-
A cyclin-dependent kinase, CDK11/p58, represses cap-dependent translation during mitosis
Cellular and Molecular Life Sciences (2020)
-
hnRNP A1/A2 and Sam68 collaborate with SRSF10 to control the alternative splicing response to oxaliplatin-mediated DNA damage
Scientific Reports (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.