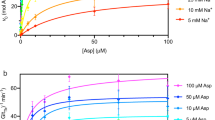
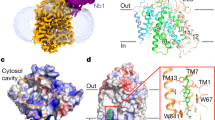
Abstract
Secondary transporters are integral membrane proteins that catalyse the movement of substrate molecules across the lipid bilayer by coupling substrate transport to one or more ion gradients, thereby providing a mechanism for the concentrative uptake of substrates. Here we describe crystallographic and thermodynamic studies of GltPh, a sodium (Na+)-coupled aspartate transporter, defining sites for aspartate, two sodium ions and d,l-threo-β-benzyloxyaspartate, an inhibitor. We further show that helical hairpin 2 is the extracellular gate that controls access of substrate and ions to the internal binding sites. At least two sodium ions bind in close proximity to the substrate and these sodium-binding sites, together with the sodium-binding sites in another sodium-coupled transporter, LeuT, define an unwound α-helix as the central element of the ion-binding motif, a motif well suited to the binding of sodium and to participation in conformational changes that accompany ion binding and unbinding during the transport cycle.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hille, B. Ion Channels of Excitable Membranes (Sinauer Associates, Sunderland, Massachusetts, 2001)
Läuger, P. Electrogenic Ion Pumps (Sinauer Associates, Sunderland, Massachusetts, 1991)
Quick, M. W. Transmembrane Transporters (Wiley-Liss, Hoboken, New Jersey, 2002)
Sobczak, I. & Lolkema, J. S. Structural and mechanistic diversity of secondary transporters. Curr. Opin. Microbiol. 8, 161–167 (2005)
Chen, N. H., Reith, M. E. & Quick, M. W. Synaptic uptake and beyond: the sodium- and chloride-dependent neurotransmitter transporter family SLC6. Pflugers Arch. 447, 519–531 (2004)
Wright, E. M. & Turk, E. The sodium/glucose cotransport family SLC5. Pflugers Arch. 447, 510–518 (2004)
Wilson, T. H. & Ding, P. Z. Sodium-substrate cotransport in bacteria. Biochim. Biophys. Acta 1505, 121–130 (2001)
Grewer, C. & Rauen, T. Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling. J. Membr. Biol. 203, 1–20 (2005)
Slotboom, D. J., Konings, W. N. & Lolkema, J. S. Structural features of the glutamate transporter family. Microbiol. Mol. Biol. Rev. 63, 293–307 (1999)
Kanner, B. I. & Bendahan, A. Binding order of substrates to the sodium and potassium ion coupled L-glutamatic acid transporter from rat brain. Biochemistry 21, 6327–6330 (1982)
Zerangue, N. & Kavanaugh, M. P. Flux coupling in a neuronal glutamate transporter. Nature 383, 634–637 (1996)
Levy, L. M., Warr, O. & Attwell, D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J. Neurosci. 18, 9620–9628 (1998)
Yernool, D., Boudker, O., Jin, Y. & Gouaux, E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature 431, 811–818 (2004)
Grewer, C. et al. Individual subunits of the glutamate transporter EAAC1 homotrimer function independently of each other. Biochemistry 44, 11913–11923 (2005)
Koch, H. P. & Larsson, H. P. Small-scale molecular motions accomplish glutamate uptake in human glutamate transporters. J. Neurosci. 25, 1730–1736 (2005)
Yamashita, A., Singh, S. K., Kawate, T., Jin, Y. & Gouaux, E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature 437, 215–223 (2005)
Grunewald, M., Bendahan, A. & Kanner, B. I. Biotinylation of single cysteine mutants of the glutamate transporter GLT-1 from rat brain reveals its unusual topology. Neuron 21, 623–632 (1998)
Slotboom, D. J., Lolkema, J. S. & Konings, W. N. Membrane topology of the C-terminal half of the neuronal, glial, and bacterial glutamate transporter family. J. Biol. Chem. 271, 31317–31321 (1996)
Seal, R. P. & Amara, S. G. A reentrant loop domain in the glutamate carrier EAAT1 participates in substrate binding and translocation. Neuron 21, 1487–1498 (1998)
Slotboom, D. J., Sobczak, I., Konings, W. N. & Lolkema, J. S. A conserved serine-rich stretch in the glutamate transporter family forms a substrate-sensitive reentrant loop. Proc. Natl Acad. Sci. USA 96, 14282–14287 (1999)
Grunewald, M. & Kanner, B. I. The accessibility of a novel reentrant loop of the glutamate transporter GLT-1 is restricted by its substrate. J. Biol. Chem. 275, 9684–9689 (2000)
Slotboom, D. J., Konings, W. N. & Lolkema, J. S. Cysteine-scanning mutagenesis reveals a highly amphipathic, pore-lining membrane-spanning helix in the glutamate transporter GltT. J. Biol. Chem. 276, 10775–10781 (2001)
Grunewald, M., Menaker, D. & Kanner, B. I. Cysteine-scanning mutagenesis reveals a conformationally sensitive reentrant pore-loop in the glutamate transporter GLT-1. J. Biol. Chem. 277, 26074–26080 (2002)
Shimamoto, K. et al. DL-threo-β-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol. Pharmacol. 53, 195–201 (1998)
Kanner, B. I. & Schuldiner, S. Mechanism of transport and storage of neurotransmitters. CRC Crit. Rev. Biochem. 22, 1–38 (1987)
Nicholls, D. & Attwell, D. The release and uptake of excitatory amino acids. Trends Pharmacol. Sci. 11, 462–468 (1990)
Arriza, J. L. et al. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J. Neurosci. 14, 5559–5569 (1994)
Slotboom, D. J., Konings, W. N. & Lolkema, J. S. Glutamate transporters combine transporter- and channel-like features. Trends Biochem. Sci. 26, 534–539 (2001)
Engelke, T., Jording, D., Kapp, D. & Pühler, A. Identification and sequence analysis of the Rhizobium meliloti dctA gene encoding the C4-dicarboxylate carrier. J. Bacteriol. 171, 5551–5560 (1989)
Yurgel, S. N. & Kahn, M. L. Sinorhizobium meliloti dctA mutants with partial ability to transport dicarboxylic acids. J. Bacteriol. 187, 1161–1172 (2005)
Shafqat, S. et al. Cloning and expression of a novel Na+-dependent neutral amino acid transporter structurally related to mammalian Na+/glutamate cotransporters. J. Biol. Chem. 268, 15351–15355 (1993)
Arriza, J. L. et al. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter family. J. Biol. Chem. 268, 15329–15332 (1993)
Ogawa, W., Kim, Y.-M., Mizushima, T. & Tsuchiya, T. Cloning and expression of the gene for the Na+-coupled serine transporter from Escherichia coli and characteristics of the transporter. J. Bacteriol. 180, 6749–6752 (1998)
Zerangue, N., Arriza, J. L., Amara, S. G. & Kavanaugh, M. P. Differential modulation of human glutamate transporter subtypes by arachidonic acid. J. Biol. Chem. 270, 6433–6435 (1995)
Kanner, B. I. & Sharon, I. Active transport of L-glutamate by membrane vesicles isolated from rat brain. Biochemistry 17, 3949–3953 (1978)
Mudring, A.-V. & Rieger, F. Lone pair effect in thallium(I) macrocyclic compounds. Inorg. Chem. 44, 6240–6243 (2005)
Tao, Z., Zhang, Z. & Grewer, C. Neutralization of the aspartic acid residue Asp-367, but not Asp-454, inhibits binding of Na+ to the glutamate-free form and cycling of the glutamate carrier EAAC1. J. Biol. Chem. 281, 10263–10272 (2006)
Kanner, B. I. & Borre, L. The dual-function glutamate transporters: structure and molecular characterization of the substrate binding sites. Biochim. Biophys. Acta 1555, 92–95 (2002)
Zarbiv, R., Grunewald, M., Kavanaugh, M. P. & Kanner, B. I. Cysteine scanning of the surroundings of an alkali-ion binding site of the glutamate transporter GLT-1 reveals a conformationally sensitive residue. J. Biol. Chem. 273, 14231–14237 (1998)
Zhang, Y. & Kanner, B. I. Two serine residues of the glutamate transporter GLT-1 are crucial for coupling the fluxes of sodium and the neurotransmitter. Proc. Natl Acad. Sci. USA 96, 1710–1715 (1999)
Brocke, L., Bendahan, A., Grunewald, M. & Kanner, B. I. Proximity of two oppositely oriented reentrant loops in the glutamate transporter GLT-1 indentified by paired cysteine mutagenesis. J. Biol. Chem. 277, 3985–3992 (2002)
Gaillard, I., Slotboom, D. J., Knol, J., Lolkema, J. S. & Konings, W. N. Purification and reconstitution of the glutamate carrier GltT of the thermophilic bacterium Bacillus stearothermophilus.. Biochemistry 35, 6150–6156 (1996)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
CCP4 Project. N. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D 50, 760–763 (1994)
Kleywegt, G. J. Use of non-crystallographic symmetry in protein structure refinement. Acta Crystallogr. D 52, 842–857 (1996)
Acknowledgements
We thank J. Mindell for support, and B. Hille and R. MacKinnon for constructive criticism. X-ray diffraction data were measured at beamlines X4A and X29 at the National Synchrotron Light Source and 8.2.2 at the Advanced Light Source. This work was supported by a National Research Service Award postdoctoral fellowship (D.Y.) and by the National Institutes of Health (E.G). E.G. is an Investigator with the Howard Hughes Medical Institute.
The coordinates for the lithium-bound native (NAT), TBOA-bound (TB) and sodium-bound (NA) states are deposited in the Protein Data Bank under accession codes 2NWL, 2NWW and 2NWX, respectively.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Notes, Supplementary Table S1 and Supplementary Figures S1-S5. (PDF 14807 kb)
Rights and permissions
About this article
Cite this article
Boudker, O., Ryan, R., Yernool, D. et al. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature 445, 387–393 (2007). https://doi.org/10.1038/nature05455
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05455
This article is cited by
-
Resource partitioning and amino acid assimilation in a terrestrial geothermal spring
The ISME Journal (2023)
-
Mutation in glutamate transporter homologue GltTk provides insights into pathologic mechanism of episodic ataxia 6
Nature Communications (2023)
-
Structural basis of vitamin C recognition and transport by mammalian SVCT1 transporter
Nature Communications (2023)
-
Astrocytic Glutamate Transporters and Migraine
Neurochemical Research (2023)
-
Structural insights into inhibitory mechanism of human excitatory amino acid transporter EAAT2
Nature Communications (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.