Abstract
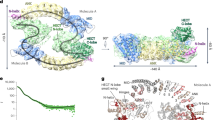
Protein phosphatase 2A (PP2A) is a principal Ser/Thr phosphatase, the deregulation of which is associated with multiple human cancers, Alzheimer’s disease and increased susceptibility to pathogen infections. How PP2A is structurally organized and functionally regulated remains unclear. Here we report the crystal structure of an AB′C heterotrimeric PP2A holoenzyme. The structure reveals that the HEAT repeats of the scaffold A subunit form a horseshoe-shaped fold, holding the catalytic C and regulatory B′ subunits together on the same side. The regulatory B′ subunit forms pseudo-HEAT repeats and interacts with the C subunit near the active site, thereby defining substrate specificity. The methylated carboxy-terminal tail of the C subunit interacts with a highly negatively charged region at the interface between A and B′ subunits, suggesting that the C-terminal carboxyl methylation of the C subunit promotes B′ subunit recruitment by neutralizing charge repulsion. Together, our structural results establish a crucial foundation for understanding PP2A assembly, substrate recruitment and regulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lin, X. H. et al. Protein phosphatase 2A is required for the initiation of chromosomal DNA replication. Proc. Natl Acad. Sci. USA 95, 14693–14698 (1998)
Depaoli-Roach, A. A. et al. Serine/threonine protein phosphatases in the control of cell function. Adv. Enzyme Regul. 34, 199–224 (1994)
Sontag, E. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell. Signal. 13, 7–16 (2001)
Janssens, V. & Goris, J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353, 417–439 (2001)
Goldberg, Y. Protein phosphatase 2A: who shall regulate the regulator?. Biochem. Pharmacol. 57, 321–328 (1999)
Virshup, D. M. Protein phosphatase 2A: a panoply of enzymes. Curr. Opin. Cell Biol. 12, 180–185 (2000)
Ogris, E., Gibson, D. M. & Pallas, D. C. Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene 15, 911–917 (1997)
Tolstykh, T., Lee, J., Vafai, S. & Stock, J. B. Carboxyl methylation regulates phosphoprotein phosphatase 2A by controlling the association of regulatory B subunits. EMBO J. 19, 5682–5691 (2000)
Wu, J. et al. Carboxyl methylation of the phosphoprotein phosphatase 2A catalytic subunit promotes its functional association with regulatory subunits in vivo.. EMBO J. 19, 5672–5681 (2000)
Yu, X. X. et al. Methylation of the protein phosphatase 2A catalytic subunit is essential for association of Bα regulatory subunit but not SG2NA, striatin, or polyomavirus middle tumor antigen. Mol. Biol. Cell 12, 185–199 (2001)
Wei, H. et al. Carboxymethylation of the PP2A catalytic subunit in Saccharomyces cerevisiae is required for efficient interaction with the B-type subunits Cdc55p and Rts1p. J. Biol. Chem. 276, 1570–1577 (2001)
Turowski, P., Fernandez, A., Favre, B., Lamb, N. J. & Hemmings, B. A. Differential methylation and altered conformation of cytoplasmic and nuclear forms of protein phosphatase 2A during cell cycle progression. J. Cell Biol. 129, 397–410 (1995)
Sontag, E. et al. Downregulation of protein phosphatase 2A carboxyl methylation and methyltransferase may contribute to Alzheimer disease pathogenesis. J. Neuropathol. Exp. Neurol. 63, 1080–1091 (2004)
Janssens, V., Goris, J. & Van Hoof, C. PP2A: the expected tumor suppressor. Curr. Opin. Genet. Dev. 15, 34–41 (2005)
Letourneux, C., Rocher, G. & Porteu, F. B56-containing PP2A dephosphorylate ERK and their activity is controlled by the early gene IEX-1 and ERK. EMBO J. 25, 727–738 (2006)
Ito, A. et al. A truncated isoform of the PP2A B56 subunit promotes cell motility through paxillin phosphorylation. EMBO J. 19, 562–571 (2000)
Seeling, J. M. et al. Regulation of β-catenin signaling by the B56 subunit of protein phosphatase 2A. Science 283, 2089–2091 (1999)
McCright, B. & Virshup, D. M. Identification of a new family of protein phosphatase 2A regulatory subunits. J. Biol. Chem. 270, 26123–26128 (1995)
Chen, W. et al. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5, 127–136 (2004)
Perrotti, D. & Neviani, P. ReSETting PP2A tumour suppressor activity in blast crisis and imatinib-resistant chronic myelogenous leukaemia. Br. J. Cancer 95, 775–781 (2006)
Goldberg, J. et al. Three-dimensional structure of the catalytic subunit of protein serine/threonine phosphatase-1. Nature 376, 745–753 (1995)
Groves, M. R., Hanlon, N., Turowski, P., Hemmings, B. A. & Barford, D. The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell 96, 99–110 (1999)
Griffith, J. P. et al. X-ray structure of calcineurin inhibited by the immunophilin-immunosuppressant FKBP12–FK506 complex. Cell 82, 507–522 (1995)
Cai, L., Chu, Y., Wilson, S. E. & Schlender, K. K. A metal-dependent form of protein phosphatase 2A. Biochem. Biophys. Res. Commun. 208, 274–279 (1995)
Kremmer, E., Ohst, K., Kiefer, J., Brewis, N. & Walter, G. Separation of PP2A core enzyme and holoenzyme with monoclonal antibodies against the regulatory A subunit: abundant expression of both forms in cells. Mol. Cell. Biol. 17, 1692–1701 (1997)
Ruediger, R. et al. Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic C subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol. Cell. Biol. 12, 4872–4882 (1992)
Ruediger, R., Pham, H. T. & Walter, G. Disruption of protein phosphatase 2A subunit interaction in human cancers with mutations in the Aα subunit gene. Oncogene 20, 10–15 (2001)
Turowski, P., Favre, B., Campbell, K. S., Lamb, N. J. & Hemmings, B. A. Modulation of the enzymatic properties of protein phosphatase 2A catalytic subunit by the recombinant 65-kDa regulatory subunit PR65α. Eur. J. Biochem. 248, 200–208 (1997)
Wang, S. S. et al. Alterations of the PPP2R1B gene in human lung and colon cancer. Science 282, 284–287 (1998)
Ruediger, R., Fields, K. & Walter, G. Binding specificity of protein phosphatase 2A core enzyme for regulatory B subunits and T antigens. J. Virol. 73, 839–842 (1999)
Ikehara, T., Shinjo, F., Ikehara, S., Imamura, S. & Yasumoto, T. Baculovirus expression, purification, and characterization of human protein phosphatase 2A catalytic subunits α and β. Protein Expr. Purif. 45, 150–156 (2006)
Philips, M. R. Methotrexate and Ras methylation: a new trick for an old drug?. Sci. STKE 2004, pe13 (2004)
Chen, J., Martin, B. L. & Brautigan, D. L. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science 257, 1261–1264 (1992)
Otwinowski, Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 (1997)
Smith, G. D. et al. The use of SnB to determine an anomalous scattering substructure. Acta Crystallogr. D 54, 799–804 (1998)
De La Fortelle, E. & Bricogne, G. Maximum-likelihood heavy-atom parameter refinement for multiple isomorphous replacement and multiwavelength anomalous diffraction methods. Methods Enzymol. 276, 472–494 (1997)
Cowtan, K. DM: an automated procedure for phase improvement by density modification. Joint CCP4 ESF-EACBM Newsl. Prot. Crystallogr. 31, 34–38 (1994)
McRee, D. E. XtalView/Xfit–A versatile program for manipulating atomic coordinates and electron density. J. Struct. Biol. 125, 156–165 (1999)
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004)
Murshudov, G. N., Vagin, A. A., Lebedev, A., Wilson, K. S. & Dodson, E. J. Efficient anisotropic refinement of macromolecular structures using FFT. Acta Crystallogr. D 55, 247–255 (1999)
Acknowledgements
We thank S. Morrone, F. Gao and other laboratory members and rotation students for help with this work. We are grateful to J. Abendroth for advice on crystallographic computation, and the staff at ALS beamline 5.0.2 for assistance with data collection. We also thank D. Virshup and X. Liu for B56 cDNAs, and N. Zheng, D. Virshup and E. Ogris for critical comments on this manuscript. This work was supported in part by an Investigator’s Award from the Burroughs Welcome Fund to W.X. and by the Keck Center for Pathogenesis at the University of Washington.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Coordinates and structure factors are deposited in the Protein Data Bank under accession code 2IAE. Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains the Supplementary Methods, Supplementary Figures 1–9 and additional references. The Supplementary Methods describes the detailed methods of protein purification, crystallization, and structural determination. (PDF 1006 kb)
Supplementary Table
This table contains the summary of crystallographic analysis. (PDF 345 kb)
Rights and permissions
About this article
Cite this article
Cho, U., Xu, W. Crystal structure of a protein phosphatase 2A heterotrimeric holoenzyme. Nature 445, 53–57 (2007). https://doi.org/10.1038/nature05351
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05351
This article is cited by
-
Cryo-EM structures of PP2A:B55–FAM122A and PP2A:B55–ARPP19
Nature (2024)
-
Genomic regulation of transcription and RNA processing by the multitasking Integrator complex
Nature Reviews Molecular Cell Biology (2023)
-
Structural mechanism for inhibition of PP2A-B56α and oncogenicity by CIP2A
Nature Communications (2023)
-
PP2A-B55: substrates and regulators in the control of cellular functions
Oncogene (2022)
-
A functional interaction between liprin-α1 and B56γ regulatory subunit of protein phosphatase 2A supports tumor cell motility
Communications Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.