Abstract
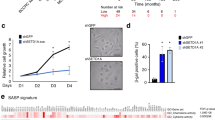
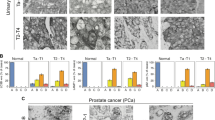
Recent studies have indicated the existence of tumorigenesis barriers that slow or inhibit the progression of preneoplastic lesions to neoplasia. One such barrier involves DNA replication stress, which leads to activation of the DNA damage checkpoint and thereby to apoptosis or cell cycle arrest1,2, whereas a second barrier is mediated by oncogene-induced senescence3,4,5,6. The relationship between these two barriers, if any, has not been elucidated. Here we show that oncogene-induced senescence is associated with signs of DNA replication stress, including prematurely terminated DNA replication forks and DNA double-strand breaks. Inhibiting the DNA double-strand break response kinase ataxia telangiectasia mutated (ATM) suppressed the induction of senescence and in a mouse model led to increased tumour size and invasiveness. Analysis of human precancerous lesions further indicated that DNA damage and senescence markers cosegregate closely. Thus, senescence in human preneoplastic lesions is a manifestation of oncogene-induced DNA replication stress and, together with apoptosis, provides a barrier to malignant progression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bartkova, J. et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434, 864–870 (2005)
Gorgoulis, V. G. et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature 434, 907–913 (2005)
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005)
Collado, M. et al. Tumour biology: senescence in premalignant tumours. Nature 436, 642 (2005)
Chen, Z. et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436, 725–730 (2005)
Braig, M. et al. Oncogene-induced senescence as an initial barrier in lymphoma development. Nature 436, 660–665 (2005)
Takai, H., Smogorzewska, A. & de Lange, T. DNA damage foci at dysfunctional telomeres. Curr. Biol. 13, 1549–1556 (2003)
d'Adda di Fagagna, F. et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature 426, 194–198 (2003)
Herbig, U., Jobling, W. A., Chen, B. P., Chen, D. J. & Sedivy, J. M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol. Cell 14, 501–513 (2004)
Di Leonardo, A., Linke, S. P., Clarkin, K. & Wahl, G. M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 8, 2540–2551 (1994)
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997)
Narita, M. et al. Rb-mediated heterochromatin formation and silencing of E2F target genes during cellular senescence. Cell 113, 703–716 (2003)
Lowe, S. W., Cepero, E. & Evan, G. Intrinsic tumour suppression. Nature 432, 307–315 (2004)
Shibuya, E. K. & Ruderman, J. V. Mos induces the in vitro activation of mitogen-activated protein kinases in lysates of frog oocytes and mammalian somatic cells. Mol. Biol. Cell 4, 781–790 (1993)
Lin, A. W. et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12, 3008–3019 (1998)
Zgheib, O. et al. ATM signaling and 53BP1. Radiother. Oncol. 76, 119–122 (2005)
Karakaidos, P. et al. Overexpression of the replication licensing regulators hCdt1 and hCdc6 characterizes a subset of non-small-cell lung carcinomas: synergistic effect with mutant p53 on tumor growth and chromosomal instability—evidence of E2F–1 transcriptional control over hCdt1. Am. J. Pathol. 165, 1351–1365 (2004)
Williams, G. H. et al. Improved cervical smear assessment using antibodies against proteins that regulate DNA replication. Proc. Natl Acad. Sci. USA 95, 14932–14937 (1998)
Vaziri, C. et al. A p53-dependent checkpoint pathway prevents rereplication. Mol. Cell 11, 997–1008 (2003)
Sarkaria, J. N. et al. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 59, 4375–4382 (1999)
Hwang, H. C. & Clurman, B. E. Cyclin E in normal and neoplastic cell cycles. Oncogene 24, 2776–2786 (2005)
Hickson, I. et al. Identification and characterization of a novel and specific inhibitor of the ataxia-telangiectasia mutated kinase ATM. Cancer Res. 64, 9152–9159 (2004)
Lambert, S. & Carr, A. M. Checkpoint responses to replication fork barriers. Biochimie 87, 591–602 (2005)
Di Micco, R. et al. Oncogene-induced senescence is a DNA damage checkpoint response triggered by DNA hyper-replication. Nature (in the press).
Katsanakis, K. D., Gorgoulis, V., Papavassiliou, A. G. & Zoumpourlis, V. K. The progression in the mouse skin carcinogenesis model correlates with ERK1/2 signaling. Mol. Med. 8, 624–637 (2002)
Raderschall, E., Golub, E. I. & Haaf, T. Nuclear foci of mammalian recombination proteins are located at single-stranded DNA regions formed after DNA damage. Proc. Natl Acad. Sci. USA 96, 1921–1926 (1999)
Ferbeyre, G. et al. PML is induced by oncogenic ras and promotes premature senescence. Genes Dev. 14, 2015–2027 (2000)
Merrick, C. J., Jackson, D. & Diffley, J. F. Visualization of altered replication dynamics after DNA damage in human cells. J. Biol. Chem. 279, 20067–20075 (2004)
Lundin, C. et al. Methyl methanesulfonate (MMS) produces heat-labile DNA damage but no detectable in vivo DNA double-strand breaks. Nucleic Acids Res. 33, 3799–3811 (2005)
Brummelkamp, T. R., Bernards, R. & Agami, R. A system for stable expression of short interfering RNAs in mammalian cells. Science 296, 550–553 (2002)
Acknowledgements
We thank Z. Lygerou and S. Taraviras for advice and reagents and M. Sideridou, N. Youroukos and M.-H. Lee for technical assistance. This work was supported by the Danish Cancer Society, the Danish National Research Foundation and the European Commission ‘Active p53’ and ‘Mutant p53’ Integrated Projects (J.B.); the National Cancer Institute, USA and the Swiss National Foundation (T.D.H.); the UICC (T.D.H. and V.G.G.); the Greek General Secretariat of Technology PENED program (V.G.G.); and the Swedish Cancer Society and Swedish Pain Relief Foundation (T.H.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Supplementary information
Supplementary Figures
This file contains Supplementary Figures 1–18. (DOC 58 kb)
Supplementary Figure Legends
This file contains text to accompany the above Supplementary Figures. (PDF 3171 kb)
Rights and permissions
About this article
Cite this article
Bartkova, J., Rezaei, N., Liontos, M. et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature 444, 633–637 (2006). https://doi.org/10.1038/nature05268
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature05268
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.