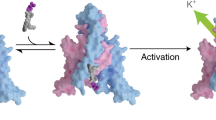
Abstract
Voltage-gated ion channels in excitable nerve, muscle, and endocrine cells generate electric signals in the form of action potentials1. However, they are also present in non-excitable eukaryotic cells and prokaryotes, which raises the question of whether voltage-gated channels might be activated by means other than changing the voltage difference between the solutions separated by the plasma membrane. The search for so-called voltage-gated channel activators is motivated in part by the growing importance of such agents in clinical pharmacology. Here we report the apparent activation of voltage-gated K+ (Kv) channels by a sphingomyelinase.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hille, B. Ion Channels of Excitable Membranes 3rd edn (Sinauer, Sunderland, Massachusetts, 2001)
Frech, G. C., VanDongen, A. M., Schuster, G., Brown, A. M. & Joho, R. H. A novel potassium channel with delayed rectifier properties isolated from rat brain by expression cloning. Nature 340, 642–645 (1989)
Aggarwal, S. K. Analysis of the Voltage-sensor in a Voltage-activated Potassium Channel. PhD thesis, Harvard Univ. (1996)
Ramos-Cerrillo, B. et al. Genetic and enzymatic characterization of sphingomyelinase D isoforms from the North American fiddleback spiders Loxosceles boneti and Loxosceles reclusa. Toxicon 44, 507–514 (2004)
Kurpiewski, G., Forrester, L. J., Barrett, J. T. & Campbell, B. J. Platelet aggregation and sphingomyelinase D activity of a purified toxin from the venom of Loxosceles reclusa. Biochim. Biophys. Acta 678, 467–476 (1981)
Rees, R. S., Nanney, L. B., Yates, R. A. & King, L. E. Jr. Interaction of brown recluse spider venom on cell membranes: the inciting mechanism? J. Invest. Dermatol. 83, 270–275 (1984)
Soucek, A., Michalec, C. & Souckova, A. Identification and characterization of a new enzyme of the group ‘phospholipase D’ isolated from Corynebacterium ovis. Biochim. Biophys. Acta 227, 116–128 (1971)
Barksdale, L., Linder, R., Sulea, I. T. & Pollice, M. Phospholipase D activity of Corynebacterium pseudotuberculosis (Corynebacterium ovis) and Corynebacterium ulcerans, a distinctive marker within the genus Corynebacterium. J. Clin. Microbiol. 13, 335–343 (1981)
Bernheimer, A. W., Campbell, B. J. & Forrester, L. J. Comparative toxinology of Loxosceles reclusa and Corynebacterium pseudotuberculosis. Science 228, 590–591 (1985)
Lee, S. & Lynch, K. R. Brown recluse spider (Loxosceles reclusa) venom phospholipase D (PLD) generates lysophosphatidic acid (LPA). Biochem. J. 391, 317–323 (2005)
de Andrade, S. A. et al. Conformational changes of Loxosceles venom sphingomyelinases monitored by circular dichroism. Biochem. Biophys. Res. Commun. 327, 117–123 (2005)
de Andrade, S. A., Murakami, M. T., Cavalcante, D. P., Arni, R. K. & Tambourgi, D. V. Kinetic and mechanistic characterization of the sphingomyelinases D from Loxosceles intermedia spider venom. Toxicon 47, 380–386 (2006)
van Meeteren, L. A. et al. Spider and bacterial sphingomyelinases D target cellular lysophosphatidic acid receptors by hydrolyzing lysophosphatidylcholine. J. Biol. Chem. 279, 10833–10836 (2004)
Hilgemann, D. W., Feng, S. & Nasuhoglu, C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE 2001, RE19 (2001)
Murakami, M. T., Fernandes-Pedrosa, M. F., Tambourgi, D. V. & Arni, R. K. Structural basis for metal ion coordination and the catalytic mechanism of sphingomyelinases D. J. Biol. Chem. 280, 13658–13664 (2005)
Bell, J. E. & Miller, C. Effects of phospholipid surface charge on ion conduction in the K+ channel of sarcoplasmic reticulum. Biophys. J. 45, 279–287 (1984)
Moczydlowski, E., Alvarez, O., Vergara, C. & Latorre, R. Effect of phospholipid surface charge on the conductance and gating of a Ca2+-activated K+ channel in planar lipid bilayers. J. Membr. Biol. 83, 273–282 (1985)
Valiyaveetil, F. I., Zhou, Y. & MacKinnon, R. Lipids in the structure, folding, and function of the KcsA K+ channel. Biochemistry 41, 10771–10777 (2002)
Bezanilla, F. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80, 555–592 (2000)
Long, S. B., Campbell, E. B. & MacKinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 309, 897–903 (2005)
Swartz, K. J. & MacKinnon, R. An inhibitor of the Kv2.1 potassium channel isolated from the venom of a Chilean tarantula. Neuron 15, 941–949 (1995)
Phillips, L. R. et al. Voltage-sensor activation with a tarantula toxin as cargo. Nature 436, 857–860 (2005)
Garcia, M. L., Garcia-Calvo, M., Hidalgo, P., Lee, A. & MacKinnon, R. Purification and characterization of three inhibitors of voltage-dependent K+ channels from Leiurus quinquestriatus var. hebraeus venom. Biochemistry 33, 6834–6839 (1994)
Frankenhaeuser, B. & Hodgkin, A. L. The action of calcium on the electrical properties of squid axons. J. Physiol. (Lond.) 137, 218–244 (1957)
Robertson, G. A., Warmke, J. M. & Ganetzky, B. Potassium currents expressed from Drosophila and mouse eag cDNAs in Xenopus oocytes. Neuropharmacology 35, 841–850 (1996)
Butler, A., Tsunoda, S., McCobb, D. P., Wei, A. & Salkoff, L. mSlo, a complex mouse gene encoding ‘maxi’ calcium-activated potassium channels. Science 261, 221–224 (1993)
Hoshi, T., Zagotta, W. N. & Aldrich, R. W. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science 250, 533–538 (1990)
Perozo, E., MacKinnon, R., Bezanilla, F. & Stefani, E. Gating currents from a nonconducting mutant reveal open-closed conformations in Shaker K+ channels. Neuron 11, 353–358 (1993)
Stith, B. J. et al. Quantification of major classes of Xenopus phospholipids by high performance liquid chromatography with evaporative light scattering detection. J. Lipid Res. 41, 1448–1454 (2000)
Lu, Z., Klem, A. M. & Ramu, Y. Ion conduction pore is conserved among potassium channels. Nature 413, 809–813 (2001)
Acknowledgements
We thank K. Lynch (University of Virginia) for sharing the cDNA clone of Lr2 isoform of SMase D; R. Joho and K. Swartz for Kv2.1 cDNA; G. Robertson and B. Ganetzky for mEAG cDNA; L. Salkoff and F. Horrigan for mSlo cDNA; K. Swartz for Shaker-IR cDNA in the pGEM-HESS vector and the HaTx sample; C.-X. Yuan for LC–MS/MS sequencing; J. R. Martinez-Francois for chemical structure drawing; R. MacKinnon for comments; and P. De Weer for review and discussion of our manuscript. This study was supported by a grant from the National Institute of General Medical Sciences to Z.L.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
LCMS/MS-identified peptide sequences match those of Lr1 and Lr2 isoforms of SMase D. (JPG 62 kb)
Rights and permissions
About this article
Cite this article
Ramu, Y., Xu, Y. & Lu, Z. Enzymatic activation of voltage-gated potassium channels. Nature 442, 696–699 (2006). https://doi.org/10.1038/nature04880
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04880
This article is cited by
-
Tracking Membrane Protein Dynamics in Real Time
The Journal of Membrane Biology (2021)
-
TRP Channels, Conformational Flexibility, and the Lipid Membrane
The Journal of Membrane Biology (2020)
-
Bacterial Sphingomyelinase is a State-Dependent Inhibitor of the Cystic Fibrosis Transmembrane conductance Regulator (CFTR)
Scientific Reports (2017)
-
Comparative study of the structure and interaction of the pore helices of the hERG and Kv1.5 potassium channels in model membranes
European Biophysics Journal (2017)
-
Membrane Protein Structure, Function, and Dynamics: a Perspective from Experiments and Theory
The Journal of Membrane Biology (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.