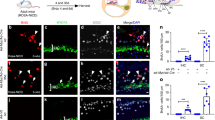
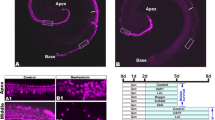
Abstract
Sensory hair cells of the mammalian organ of Corti in the inner ear do not regenerate when lost as a consequence of injury, disease, or age-related deafness. This contrasts with other vertebrates such as birds, where the death of hair cells causes surrounding supporting cells to re-enter the cell cycle and give rise to both new hair cells and supporting cells1,2. It is not clear whether the lack of mammalian hair cell regeneration is due to an intrinsic inability of supporting cells to divide and differentiate or to an absence or blockade of regenerative signals. Here we show that post-mitotic supporting cells3 purified from the postnatal mouse cochlea retain the ability to divide and trans-differentiate into new hair cells in culture. Furthermore, we show that age-dependent changes in supporting cell proliferative capacity are due in part to changes in the ability to downregulate the cyclin-dependent kinase inhibitor p27Kip1 (also known as Cdkn1b). These results indicate that postnatal mammalian supporting cells are potential targets for therapeutic manipulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Corwin, J. T. & Cotanche, D. A. Regeneration of sensory hair cells after acoustic trauma. Science 240, 1772–1774 (1988)
Ryals, B. M. & Rubel, E. W. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 240, 1774–1776 (1988)
Ruben, R. J. Development of the inner ear of the mouse: a radioautographic study of terminal mitoses. Acta Otolaryngol. Suppl. 220, 1–44 (1967)
Zine, A. et al. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J. Neurosci. 21, 4712–4720 (2001)
Lewis, A. K., Frantz, G. D., Carpenter, D. A., de Sauvage, F. & Gao, W. Q. Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mech. Dev. 78, 159–163 (1998)
Chen, P. & Segil, N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 126, 1581–1590 (1999)
Coppens, A. G., Kiss, R., Heizmann, C. W., Schafer, B. W. & Poncelet, L. Immunolocalization of the calcium binding S100A1, S100A5 and S100A6 proteins in the dog cochlea during postnatal development. Brain Res. Dev. Brain Res. 126, 191–199 (2001)
Bermingham, N. A. et al. Math1: an essential gene for the generation of inner ear hair cells. Science 284, 1837–1841 (1999)
Erkman, L. et al. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature 381, 603–606 (1996)
Doetzlhofer, A., White, P. M., Johnson, J. E., Segil, N. & Groves, A. K. In vitro growth and differentiation of mammalian sensory hair cell progenitors: a requirement for EGF and periotic mesenchyme. Dev. Biol. 272, 432–447 (2004)
Roberson, D. W., Kreig, C. S. & Rubel, E. W. Light microscopic evidence that direct transdifferentiation gives rise to new hair cells in regenerating avian auditory epithelium. Audit. Neurosci. 2, 195–205 (1996)
Hasson, T. et al. Unconventional myosins in inner-ear sensory epithelia. J. Cell Biol. 1997, 1287–1307 (1997)
Gestwa, G. et al. Differential expression of trkB.T1 and trkB.T2, truncated trkC, and p75(NGFR) in the cochlea prior to hearing function. J. Comp. Neurol. 414, 33–49 (1999)
Helms, A. W., Abney, A. L., Ben-Arie, N., Zoghbi, H. Y. & Johnson, J. E. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development 127, 1185–1196 (2000)
Chen, P., Johnson, J. E., Zoghbi, H. Y. & Segil, N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 129, 2495–2505 (2002)
Colvin, J. S., Bohne, B. A., Harding, G. W., McEwen, D. G. & Ornitz, D. M. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nature Genet. 12, 390–397 (1996)
Lim, D. J. & Anniko, M. Developmental morphology of the mouse inner ear. A scanning electron microscopic observation. Acta Otolaryngol. Suppl. 422, 1–69 (1985)
Dechesne, C. J., Rabejac, D. & Desmadryl, G. Development of calretinin immunoreactivity in the mouse inner ear. J. Comp. Neurol. 346, 517–529 (1994)
Zheng, L. et al. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell 102, 377–385 (2000)
Meyers, J. R. et al. Lighting up the senses: FM1–43 loading of sensory cells through nonselective ion channels. J. Neurosci. 23, 4054–4065 (2003)
Pujol, R. & Hilding, D. Anatomy and physiology of the onset of auditory function. Acta Otolaryngol. 76, 1–10 (1973)
Chardin, S. & Romand, R. Regeneration and mammalian auditory hair cells. Science 267, 707–709 (1995)
Izumikawa, M. et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nature Med. 11, 271–276 (2005)
Zheng, J. L. & Gao, W. Q. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nature Neurosci. 3, 580–586 (2000)
Warchol, M. E. & Corwin, J. T. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J. Neurosci. 16, 5466–5477 (1996)
Stone, J. S. & Rubel, E. W. Cellular studies of auditory hair cell regeneration in birds. Proc. Natl Acad. Sci. 97, 11714–11721 (2000)
Yang, X. M., Model, P. & Heintz, N. Homologous recombination-based modification in Escherichia coli and germline transmission in transgenic mice of a bacterial artificial chromosome. Nature Biotechnol. 15, 859–865 (1997)
Doetzlhofer, A., White, P. M., Lee, Y. S., Groves, A. & Segil, N. Prospective identification and purification of hair cell and supporting cell progenitors from the embryonic cochlea. Brain Res. published online 6 April 2006 (doi:PMID:16616734).
Fero, M. L. et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27(Kip1)-deficient mice. Cell 85, 733–744 (1996)
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(- ΔΔC(T)) method. Methods 25, 402–408 (2001)
Acknowledgements
We gratefully acknowledge S. Juntilla, J. Llamas and W. Makmura for animal care and genotyping; S. Chavira at the USC Flow Cytometry Section for FACS assistance, the Transgenic Mouse Core Facility at USC for generating the p27–GFP BAC transgenics, A. J. Hudspeth for the anti-espin antibody, T. Hasson for anti-myosin-VI and anti-myosin-VIIa, J. Johnson for Math1-GFP mice, J. Roberts for the p27Kip1-/- mice, and E. W. Rubel for helpful advice. This work was supported by grants from the Hair Cell Regeneration Initiative of the National Organisation for Hearing Research and from the NIH. P.M.W. was supported by an NIH training grant. Author contributions The project was conceived by A.K.G. and N.S.; experiments were planned, performed and analysed jointly by A.D. and P.M.W. with advice from A.K.G. and N.S. The p27–gfp transgenic mouse was constructed by Y.S.L.; the paper was written by A.D., A.K.G., N.S. and P.M.W.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Text, Supplementary Figures 1–3, Supplementary Table, Supplementary Methods and Supplementary Table. (PDF 1383 kb)
Rights and permissions
About this article
Cite this article
White, P., Doetzlhofer, A., Lee, Y. et al. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature 441, 984–987 (2006). https://doi.org/10.1038/nature04849
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04849
This article is cited by
-
Effect of ursodiol on alginate/PLL nanoparticles with non-ionic surfactant for gene delivery
Journal of Nanoparticle Research (2024)
-
AAV-Net1 facilitates the trans-differentiation of supporting cells into hair cells in the murine cochlea
Cellular and Molecular Life Sciences (2023)
-
The proper timing of Atoh1 expression is pivotal for hair cell subtype differentiation and the establishment of inner ear function
Cellular and Molecular Life Sciences (2023)
-
Transcriptomic and epigenomic analyses explore the potential role of H3K4me3 in neomycin-induced cochlear Lgr5+ progenitor cell regeneration of hair cells
Human Cell (2022)
-
The interaction of Notch and Wnt signaling pathways in vertebrate regeneration
Cell Regeneration (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.