Abstract
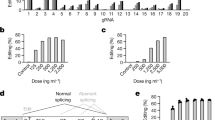
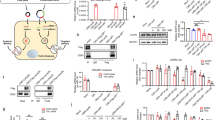
The opportunity to harness the RNA interference (RNAi) pathway to silence disease-causing genes holds great promise for the development of therapeutics directed against targets that are otherwise not addressable with current medicines1,2. Although there are numerous examples of in vivo silencing of target genes after local delivery of small interfering RNAs (siRNAs)3,4,5, there remain only a few reports of RNAi-mediated silencing in response to systemic delivery of siRNA6,7,8, and there are no reports of systemic efficacy in non-rodent species. Here we show that siRNAs, when delivered systemically in a liposomal formulation, can silence the disease target apolipoprotein B (ApoB) in non-human primates. APOB-specific siRNAs were encapsulated in stable nucleic acid lipid particles (SNALP) and administered by intravenous injection to cynomolgus monkeys at doses of 1 or 2.5 mg kg-1. A single siRNA injection resulted in dose-dependent silencing of APOB messenger RNA expression in the liver 48 h after administration, with maximal silencing of >90%. This silencing effect occurred as a result of APOB mRNA cleavage at precisely the site predicted for the RNAi mechanism. Significant reductions in ApoB protein, serum cholesterol and low-density lipoprotein levels were observed as early as 24 h after treatment and lasted for 11 days at the highest siRNA dose, thus demonstrating an immediate, potent and lasting biological effect of siRNA treatment. Our findings show clinically relevant RNAi-mediated gene silencing in non-human primates, supporting RNAi therapeutics as a potential new class of drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Novina, C. D. & Sharp, P. A. The RNAi revolution. Nature 430, 161–164 (2004)
Shankar, P., Manjunath, N. & Lieberman, J. The prospect of silencing disease using RNA interference. J. Am. Med. Assoc. 293, 1367–1373 (2005)
Thakker, D. R. et al. Neurochemical and behavioral consequences of widespread gene knockdown in the adult mouse brain by using nonviral RNA interference. Proc. Natl Acad. Sci. USA 101, 17270–17275 (2004)
Bitko, V., Musiyenko, A., Shulyayeva, O. & Barik, S. Inhibition of respiratory viruses by nasally administered siRNA. Nature Med. 11, 50–55 (2005)
Palliser, D. et al. An siRNA-based microbicide protects mice from lethal herpes simplex virus 2 infection. Nature 439, 89–94 (2006)
Soutschek, J. et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature 432, 173–178 (2004)
Song, E. et al. Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nature Biotechnol. 23, 709–717 (2005)
Morrissey, D. V. et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nature Biotechnol. 23, 1002–1007 (2005)
Brown, M. S. & Goldstein, J. L. A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34–47 (1986)
Cannon, C. P. et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N. Engl. J. Med. 350, 1495–1504 (2004)
Ridker, P. M. et al. C-reactive protein levels and outcomes after statin therapy. N. Engl. J. Med. 352, 20–28 (2005)
Crooke, R. M. et al. An apolipoprotein B antisense oligonucleotide lowers LDL cholesterol in hyperlipidemic mice without causing hepatic steatosis. J. Lipid Res. 46, 872–884 (2005)
Song, E. et al. Sustained small interfering RNA-mediated human immunodeficiency virus type 1 inhibition in primary macrophages. J. Virol. 77, 7174–7181 (2003)
Bartlett, D. W. & Davis, M. E. Insights into the kinetics of siRNA-mediated gene silencing from live-cell and live-animal bioluminescent imaging. Nucleic Acids Res. 34, 322–333 (2006)
Lennernas, H. & Fager, G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. Similarities and differences. Clin. Pharmacokinet. 32, 403–425 (1997)
Levin, A. A. A review of the issues in the pharmacokinetics and toxicology of phosphorothioate antisense oligonucleotides. Biochim. Biophys. Acta 1489, 69–84 (1999)
Chonn, A., Cullis, P. R. & Devine, D. V. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J. Immunol. 146, 4234–4241 (1991)
Hornung, V. et al. Sequence-specific potent induction of IFN-α by short interfering RNA in plasmacytoid dendritic cells through TLR7. Nature Med. 11, 263–270 (2005)
Judge, A. D. et al. Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nature Biotechnol. 23, 457–462 (2005)
Acknowledgements
We are grateful to P. Sharp, J. Maraganore and N. Mahanthappa for their assistance and support in this study. We would also like to thank W. J. Schneider, J. Frohlich, M. Hayden and J. E. Vance for discussions. We acknowledge the technical assistance of C. Woppmann and A. Wetzel, and thank V. Kesavan and G. Wang for preparation of the cholesterol-conjugated siRNA used in this study. Finally, we thank S. Young for providing anti-ApoB antibodies. This work was supported by grants from the National Science and Engineering Research Council of Canada (to A.J.W. and M.N.F.). Author Contributions This work represents the outcome of a collaboration between scientists at Alnylam Pharmaceuticals and Protiva Biotherapeutics Inc.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors of this paper are employees of either Alnylam Pharmaceuticals or Protiva Biotherapeutics Inc., and therefore declare competing financial interests.
Supplementary information
Supplementary Methods
This file contains details of experimental methods used in this study. (PDF 37 kb)
Supplementary Figures and Table
This file contains Supplementary Figures 1–6 with their legends and Supplementary Table 1. (PDF 127 kb)
Rights and permissions
About this article
Cite this article
Zimmermann, T., Lee, A., Akinc, A. et al. RNAi-mediated gene silencing in non-human primates. Nature 441, 111–114 (2006). https://doi.org/10.1038/nature04688
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature04688
This article is cited by
-
RNA interference in the era of nucleic acid therapeutics
Nature Biotechnology (2024)
-
Nanoparticle-mediated targeting of the fusion gene RUNX1/ETO in t(8;21)-positive acute myeloid leukaemia
Leukemia (2023)
-
In vivo PAR-CLIP (viP-CLIP) of liver TIAL1 unveils targets regulating cholesterol synthesis and secretion
Nature Communications (2023)
-
Synthesis of siRNA nanoparticles to silence plaque-destabilizing gene in atherosclerotic lesional macrophages
Nature Protocols (2022)
-
Nanoparticles in the diagnosis and treatment of vascular aging and related diseases
Signal Transduction and Targeted Therapy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.