Abstract
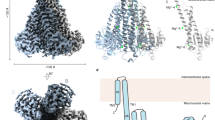
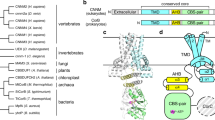
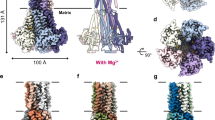
The magnesium ion, Mg2+, is essential for myriad biochemical processes and remains the only major biological ion whose transport mechanisms remain unknown. The CorA family of magnesium transporters is the primary Mg2+ uptake system of most prokaryotes1,2,3 and a functional homologue of the eukaryotic mitochondrial magnesium transporter4. Here we determine crystal structures of the full-length Thermotoga maritima CorA in an apparent closed state and its isolated cytoplasmic domain at 3.9 Å and 1.85 Å resolution, respectively. The transporter is a funnel-shaped homopentamer with two transmembrane helices per monomer. The channel is formed by an inner group of five helices and putatively gated by bulky hydrophobic residues. The large cytoplasmic domain forms a funnel whose wide mouth points into the cell and whose walls are formed by five long helices that are extensions of the transmembrane helices. The cytoplasmic neck of the pore is surrounded, on the outside of the funnel, by a ring of highly conserved positively charged residues. Two negatively charged helices in the cytoplasmic domain extend back towards the membrane on the outside of the funnel and abut the ring of positive charge. An apparent Mg2+ ion was bound between monomers at a conserved site in the cytoplasmic domain, suggesting a mechanism to link gating of the pore to the intracellular concentration of Mg2+.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nelson, D. L. & Kennedy, E. P. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J. Biol. Chem. 246, 3042–3049 (1971)
Hmiel, S. P., Snavely, M. D., Miller, C. G. & Maguire, M. E. Magnesium transport in Salmonella typhimurium: Characterization of magnesium influx and cloning of a transport gene. J. Bacteriol. 168, 1444–1450 (1986)
Bui, D. M., Gregan, J., Jarosch, E., Ragnini, A. & Schweyen, R. J. The bacterial magnesium transporter CorA can functionally substitute for its putative homologue Mrs2p in the yeast inner mitochondrial membrane. J. Biol. Chem. 274, 20438–20443 (1999)
Kehres, D. G. & Maguire, M. E. Structure, properties and regulation of magnesium transport proteins. Biometals 15, 261–270 (2002)
Kehres, D. G., Lawyer, C. H. & Maguire, M. E. The CorA magnesium transporter gene family. Microb. Compar. Genom. 43, 151–169 (1998)
Worlock, A. J. & Smith, R. L. ZntB is a novel Zn2+ transporter in Salmonella enterica serovar Typhimurium. J. Bacteriol. 184, 4369–4373 (2002)
Gardner, R. C. Genes for magnesium transport. Curr. Opin. Plant Biol. 6, 263–267 (2003)
Cordes, F. S., Bright, J. N. & Sansom, M. S. Proline-induced distortions of transmembrane helices. J. Mol. Biol. 323, 951–960 (2002)
Tieleman, D. P., Shrivastava, I. H., Ulmschneider, M. R. & Sansom, M. S. Proline-induced hinges in transmembrane helices: possible roles in ion channel gating. Proteins 44, 63–72 (2001)
Knoop, V., Groth-Malonek, M., Gebert, M., Eifler, K. & Weyand, K. Transport of magnesium and other divalent cations: evolution of the 2-TM-GxN proteins in the MIT superfamily. Mol. Genet. Genom. 274, 205–216 (2005)
Doyle, D. A. Molecular insights into ion channel function. Mol. Membr. Biol. 21, 221–225 (2004)
Roux, B. & MacKinnon, R. The cavity and pore helices in the KcsA K+ channel: electrostatic stabilization of monovalent cations. Science 285, 100–102 (1999)
Doyle, D. A. et al. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science 280, 69–77 (1998)
Kuo, A. et al. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science 300, 1922–1926 (2003)
Chang, G., Spencer, R. H., Lee, A. T., Barclay, M. T. & Rees, D. C. Structure of the MscL homolog from Mycobacterium tuberculosis: A gated mechanosensitive ion channel. Science 282, 2220–2226 (1998)
Miyazawa, A., Fujiyoshi, Y. & Unwin, N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 423, 949–955 (2003)
MacKinnon, R. Potassium channels and the atomic basis for selective ion conduction. Biosci. Rep. 24, 75–100 (2004)
Maguire, M. E. & Cowan, J. A. Mg2+ chemistry and biochemistry. Biometals 15, 203–210 (2002)
Snavely, M. D., Florer, J. B., Miller, C. G. & Maguire, M. E. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J. Bacteriol. 171, 4761–4766 (1989)
Kucharski, L. M., Lubbe, W. J. & Maguire, M. E. Cation hexaammines are selective and potent inhibitors of the CorA magnesium transport system. J. Biol. Chem. 275, 16767–16773 (2000)
Szegedy, M. A. & Maguire, M. E. The CorA Mg2+ transport protein of Salmonella typhimurium. Mutagenesis of conserved residues in the second membrane domain. J. Biol. Chem. 274, 36973–36979 (1999)
Langosch, D., Thomas, L. & Betz, H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc. Natl Acad. Sci. USA 85, 7394–7398 (1988)
Lester, H. A. The permeation pathway of neurotransmitter-gated ion channels. Annu. Rev. Biophys. Biomol. Struct. 21, 267–292 (1992)
Delano, W. L. PyMOLhttp://pymol.sourceforge.net/ (cited 19 October 2005).
Korolev, S. et al. The crystal structure of spermidine synthase with a multisubstrate adduct inhibitor. Nature Struct. Biol. 9, 27–31 (2002)
Acknowledgements
The research was supported by grants from Genome Canada, the National Institutes of Health, the US Department of Energy, Office of Biological and Environmental Research, and the Structural Genomics Consortium. We thank M. McMillan and Y. Kim for assistance in collecting data, and A. Savchenko and the Indians and Red Wings screening teams for their guidance. We also thank C. Charky and D. Bouchard at Nextal Biotechnologies for their assistance throughout our membrane protein crystallization efforts.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Ribbon diagram of the CorA soluble domain. (JPG 62 kb)
Supplementary Figure 2
Close-up view of the CorA periplasmic surface. (JPG 53 kb)
Supplementary Figure 3
Secondary structure predictions of CorA homologues. (JPG 137 kb)
Supplementary Figure 4
There are over 250 CorA homologs and around 200 Mrs2 homologs listed in the current NCBI and Swiss-Prot databases. (JPG 307 kb)
Supplementary Figure 5
View of the electrostatic potential of the CorA surface. (JPG 59 kb)
Supplementary Figure Legends
This file contains legends to the Supplementary Figures. (DOC 30 kb)
Supplementary Data
This file contains Supplementary Methods, Table 1 and additional references. (DOC 60 kb)
Rights and permissions
About this article
Cite this article
Lunin, V., Dobrovetsky, E., Khutoreskaya, G. et al. Crystal structure of the CorA Mg2+ transporter. Nature 440, 833–837 (2006). https://doi.org/10.1038/nature04642
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04642
This article is cited by
-
Molecular basis of Mg2+ permeation through the human mitochondrial Mrs2 channel
Nature Communications (2023)
-
Genome-wide identification and expression analysis of magnesium transporter gene family in grape (Vitis vinifera)
BMC Plant Biology (2022)
-
Conserved mechanism for vacuolar magnesium sequestration in yeast and plant cells
Nature Plants (2022)
-
Structural and functional comparison of magnesium transporters throughout evolution
Cellular and Molecular Life Sciences (2022)
-
Crystal structure of an archaeal CorB magnesium transporter
Nature Communications (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.