Abstract
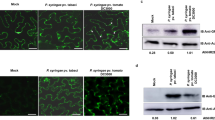
To cause diseases in plants, pathogenic microorganisms have evolved mechanisms to deliver proteins directly into plant cells, where they suppress plant defences and facilitate tissue invasion1,2,3. How plant pathogenic fungi, which cause many of the world's most serious plant diseases, deliver proteins during plant infection is currently unknown. Here we report the characterization of a P-type ATPase-encoding gene, MgAPT2, in the economically important rice blast pathogen Magnaporthe grisea, which is required for exocytosis during plant infection. Targeted gene replacement showed that MgAPT2 is required for both foliar and root infection by the fungus, and for the rapid induction of host defence responses in an incompatible reaction. ΔMgapt2 mutants are impaired in the secretion of a range of extracellular enzymes and accumulate abnormal Golgi-like cisternae. However, the loss of MgAPT2 does not significantly affect hyphal growth or sporulation, indicating that the establishment of rice blast disease involves the use of MgApt2-dependent exocytotic processes that operate during plant infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Alfano, J. R. & Collmer, A. Type III secretion system effector proteins: Double agents in bacterial disease and plant defense. Annu. Rev. Phytopathol. 42, 385–414 (2004)
Nimchuk, Z., Eulgem, T., Holt, B. E. & Dangl, J. L. Recognition and response in the plant immune system. Annu. Rev. Genet. 37, 579–609 (2003)
Ghosh, P. Process of protein transport by the type III secretion system. Microbiol. Molec. Biol. Rev. 68, 771–795 (2004)
Mendgen, K., Hahn, M. & Deising, H. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 34, 367–386 (1996)
Talbot, N. J. On the trail of a cereal killer: investigating the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 57, 177–202 (2003)
Howard, R. J., Ferrari, M. A., Roach, D. H. & Money, N. P. Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc. Natl Acad. Sci. USA 88, 11281–11284 (1991)
Sesma, A. & Osbourn, A. E. The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature 431, 582–586 (2004)
Dean, R. A. et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434, 980–986 (2005)
Chen, C.-Y., Ingram, M. F., Rosal, P. H. & Graham, T. R. Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase in yeast late Golgi function. J. Cell Biol. 147, 1223–1236 (1999)
Gall, W. E. et al. Drs2p-dependent formation of exocytotic clathrin-coated vesicles in vivo. Curr. Biol. 12, 1623–1627 (2002)
Hua, Z., Fatheddin, P. & Graham, T. R. An essential subfamily of Drs2-related P-type ATPases is required for protein trafficking between Golgi complex and endosomal/vacuolar system. Mol. Biol. Cell 13, 3162–3177 (2002)
Lutsenko, S. & Kaplan, J. H. Organization of P-type ATPases: Significance of structural diversity. Biochemistry 34, 15607–15613 (1995)
Carroll, A. M., Sweigard, J. A. & Valent, B. Improved vectors for selecting resistance to hygromycin. Fungal Genet. Newsl. 42, 22 (1994)
Pomorski, T. et al. Drs2-related P-type ATPases Dnf1p and Dnf2p are required for phospholipids translocation across the yeast plasma membrane and serve a role in endocytosis. Mol. Biol. Cell 14, 1240–1254 (2003)
Lussier, M., Sdicu, A. M., Camirand, A. & Bussey, H. Functional Characterization of the YUR1, KTR1, and KTR2 genes as members of the yeast KRE2/MNT1 mannosyltransferase gene family. J. Biol. Chem. 271, 11001–11008 (1996)
Southern, J. A., Young, D. F., Heaney, F., Baumgartner, W. & Randall, R. E. Identification of an epitope on the P and V proteins of simian virus 5 that distinguishes between two isolates with different biological characteristics. J. Gen. Virol. 72, 1551–1557 (1991)
Jia, Y., McAdams, S. A., Bryan, G. T., Hershey, H. P. & Valent, B. Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 19, 4004–4014 (2000)
Xu, J. R. & Hamer, J. E. MAP kinase and cAMP signalling regulate infection structure formation and pathogenic growth in the rice blast fungus Magnaporthe grisea. Genes Dev. 10, 2696–2706 (1996)
Kim, S. et al. Molecular characterization of the cDNA encoding an acific isoform of PR-1 protein in rice. Mol. Cells 11, 115–121 (2001)
Balhadère, P. V. & Talbot, N. J. Pde1 encodes a P-type ATPase involved in appressorium-mediate plant infection by the rice blast fungus Magnaporthe grisea. Plant Cell 13, 1987–2004 (2001)
Rose, M. D., Winston, F. & Hieter, P. Methods in Yeast Genetics: A Laboratory Course Manual (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, 1990)
Frohman, M. A., Dush, M. K. & Martin, G. R. Rapid production of full-length cDNA from rare transcripts: Amplification using a single gene-specific oligonucleotide primer. Proc. Natl Acad. Sci. USA 85, 8998–9002 (1988)
Talbot, N. J., Ebbole, D. J. & Hamer, J. E. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell 5, 1575–1590 (1993)
Valent, B., Farrall, L. & Chumley, F. G. Magnaporthe grisea genes for pathogenicity and virulence identified through a series of backcrosses. Genetics 127, 87–101 (1991)
Zeng, L. R. et al. Spotted leaf11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell 17, 2795–2808 (2004)
Chida, T. & Sisler, H. D. Restoration of appressorial penetration ability by melanin precursosrs in Pyricularia oryzae treated with antipenetrants and in melanin-deficient mutants. J. Pestic. Sci. 12, 49–55 (1987)
Sweigard, J. A., Chumley, F. G., Carroll, A., Farrall, L. & Valent, B. A series of vectors for fungal transformation. Fungal Genet. Newsl. 44, 52–53 (1997)
Tucker, S. L. et al. A fungal metallothionein is required for pathogenicity of Magnaporthe grisea. Plant Cell 16, 1575–1588 (2004)
Acknowledgements
We thank C. Hawes and B. Martin for cryofixation and freeze substitution work, G.-L. Wang for supply of IR-68 seeds and T. Graham for supplying the BY4739, PFY3273A and DS94 yeast APT mutants. This study was supported by a grant to N.J.T. from the Biological Sciences and Biotechnology Research Council (BBSRC). Author Contributions Experimental work and data analysis were performed by M.J.G. and N.J.T. C.R.T. performed all immunological work and associated data analysis. G.E.W. performed electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1.
Yeast complementation assay showing the Magnaporthe APT2 gene is unable to complement the cold sensitive phenotype of the Saccharomyces cerevisiae Δdrs2 mutant. (PDF 697 kb)
Supplementary Figure 2.
Influence of MgAPT2(p) expression on aminophospholipid internalisation in intact yeast cells. MgAPT2(p) is unable to restore the ability of drs2Δ to internalize phosphatidylserine. (PDF 193 kb)
Supplementary Figure 3.
Generation and virulence of ΔMgapt2 mutants. Targeted gene replacement strategy showing the generation of 6 gene replacement transformants. Subsequent plant infection assays and epidermal penetration assays show a significant reduction of ΔMgapt2 mutants to cause rice blast disease. (PDF 176 kb)
Supplementary Figure 4.
Electron micrographs showing the accumulation of Berkeley bodies in the yeast drs2Δ mutant. (PDF 603 kb)
Supplementary Figure 5.
The loss of MgAPT2 has no affect on endocytosis. Wild type and ΔMgapt2 conidia were stained with FM4-64 and show no significant difference in uptake kinetics. (PDF 2117 kb)
Supplementary Figure 6.
The ΔMgapt2 mutant is impaired in its ability to secrete α-amylase. Using an anti-α-amylase antibody, immunogold experiments were conducted. The resulting electron micrographs show ΔMgapt2 is severely reduced in α-amylase secretion. (PDF 1315 kb)
Supplementary Figure 7.
Plate assay showing ΔMgapt2 mutants are not compromised in their ability to secrete the enzyme trehalase. (PDF 2506 kb)
Supplementary Table 1.
Amino acid similarity and identity of MgAPT2 and the yeast aminophospholipid translocases. (PDF 42 kb)
Supplementary Table 2.
Table showing the ability of the ΔMgapt2 mutant to grow on single carbon sources. (PDF 46 kb)
Supplementary Table 3.
Summary of Magnaporthe strains used in the study. (PDF 42 kb)
Supplementary Table 4.
Summary of yeast strains used in the study. (PDF 42 kb)
Supplementary Methods.
Comprehensive and detailed explanation of protocols used in the study together with references. (PDF 145 kb)
Rights and permissions
About this article
Cite this article
Gilbert, M., Thornton, C., Wakley, G. et al. A P-type ATPase required for rice blast disease and induction of host resistance. Nature 440, 535–539 (2006). https://doi.org/10.1038/nature04567
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04567
This article is cited by
-
Ubiquitination in the rice blast fungus Magnaporthe oryzae: from development and pathogenicity to stress responses
Phytopathology Research (2022)
-
A novel ATPase gene, Ab-atps, plays an important role in the interaction of rice and white tip nematode, Aphelenchoides besseyi
Scientific Reports (2021)
-
Lipid flippases in polarized growth
Current Genetics (2021)
-
The P5-type ATPase Spf1 is required for development and virulence of the rice blast fungus Pyricularia oryzae
Current Genetics (2020)
-
Elucidation of the molecular responses of a cucumber segment substitution line carrying Pm5.1 and its recurrent parent triggered by powdery mildew by comparative transcriptome profiling
BMC Genomics (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.