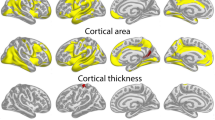
Abstract
Children who are adept at any one of the three academic ‘R's (reading, writing and arithmetic) tend to be good at the others, and grow into adults who are similarly skilled at diverse intellectually demanding activities1,2,3. Determining the neuroanatomical correlates of this relatively stable individual trait of general intelligence has proved difficult, particularly in the rapidly developing brains of children and adolescents. Here we demonstrate that the trajectory of change in the thickness of the cerebral cortex, rather than cortical thickness itself, is most closely related to level of intelligence. Using a longitudinal design, we find a marked developmental shift from a predominantly negative correlation between intelligence and cortical thickness in early childhood to a positive correlation in late childhood and beyond. Additionally, level of intelligence is associated with the trajectory of cortical development, primarily in frontal regions implicated in the maturation of intelligent activity4,5. More intelligent children demonstrate a particularly plastic cortex, with an initial accelerated and prolonged phase of cortical increase, which yields to equally vigorous cortical thinning by early adolescence. This study indicates that the neuroanatomical expression of intelligence in children is dynamic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Spearman, C. 'General intelligence' objectively determined and measured. Am. J. Psychol. 15, 201–293 (1904)
Gottfredson, L. S. Why g matters: The complexity of everyday life. Intelligence 24, 79–132 (1997)
Deary, I. J., Whalley, L. J., Lemmon, H., Crawford, J. R. & Starr, J. M. The stability of individual differences in mental ability from childhood to old age: Follow-up of the 1932 Scottish Mental Survey. Intelligence 28, 49–55 (2000)
Booth, J. R. et al. Neural development of selective attention and response inhibition. Neuroimage 20, 737–751 (2003)
Sowell, E. R. et al. Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 24, 8223–8231 (2004)
McDaniel, M. Big-brained people are smarter. Intelligence 33, 337–346 (2005)
Wilke, M., Sohn, J. H., Byars, A. W. & Holland, S. K. Bright spots: correlations of gray matter volume with IQ in a normal pediatric population. Neuroimage 20, 202–215 (2003)
Frangou, S., Chitins, X. & Williams, S. C. Mapping IQ and gray matter density in healthy young people. Neuroimage 23, 800–805 (2004)
Haier, R. J., Jung, R. E., Yeo, R. A., Head, K. & Alkire, M. T. Structural brain variation and general intelligence. Neuroimage 23, 425–433 (2004)
Kraemer, H. C., Yesavage, J. A., Taylor, J. L. & Kupfer, D. How can we learn about developmental processes from cross-sectional studies, or can we? Am. J. Psychiatry 157, 163–171 (2000)
Giedd, J. N. et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neurosci. 2, 861–863 (1999)
Wechsler, D. Manual for the Wechsler Intelligence Scale for Children—Revised (The Psychological Corporation, New York, 1974)
O'Donnell, S., Noseworthy, M. D., Levine, B. & Dennis, M. Cortical thickness of the frontopolar area in typically developing children and adolescents. Neuroimage 24, 948–954 (2005)
Kabani, N., Le Goualher, G., MacDonald, D. & Evans, A. C. Measurement of cortical thickness using an automated 3-D algorithm: a validation study. Neuroimage 13, 375–380 (2001)
Lerch, J. P. & Evans, A. C. Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage 24, 163–173 (2005)
Gray, J. R., Chabris, C. F. & Braver, T. S. Neural mechanisms of general fluid intelligence. Nature Neurosci. 6, 316–322 (2003)
Duncan, J. et al. A neural basis for general intelligence. Science 289, 457–460 (2000)
Kostovic, I., Judas, M., Rados, M. & Hrabac, P. Laminar organization of the human fetal cerebrum revealed by histochemical markers and magnetic resonance imaging. Cereb. Cortex 12, 536–544 (2002)
Kostovic, I. & Rakic, P. Developmental history of the transient subplate zone in the visual and somatosensory cortex of the macaque monkey and human brain. J. Comp. Neurol. 297, 441–470 (1990)
Yakovlev, P. I. & Lecours, A. R. in Regional Development of the Brain in Early Life (ed. Minokowski, A.) (Blackwell Scientific, Oxford, 1967)
Huttenlocher, P. R. & Dabholkar, A. S. Regional differences in synaptogenesis in human cerebral cortex. J. Comp. Neurol. 387, 167–178 (1997)
Hensch, T. K. Critical period regulation. Annu. Rev. Neurosci. 27, 549–579 (2004)
Chugani, H. T., Phelps, M. E. & Mazziotta, J. C. Positron emission tomography study of human brain functional development. Ann. Neurol. 22, 487–497 (1987)
Collins, D. L., Neelin, P., Peters, T. M. & Evans, A. C. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J. Comput. Assist. Tomogr. 18, 192–205 (1994)
Sled, J. G., Zijdenbos, A. P. & Evans, A. C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging 17, 87–97 (1998)
Zijdenbos, A. P., Forghani, R. & Evans, A. C. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans. Med. Imaging 21, 1280–1291 (2002)
MacDonald, D., Kabani, N., Avis, D. & Evans, A. C. Automated 3-D extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage 12, 340–356 (2000)
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995)
Pinheiro, J. C. & Bates, D. M. Mixed-effects Models in S and S-PLUS (Springer, New York, 2000)
Genovese, C. R., Lazar, N. A. & Nichols, T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15, 870–878 (2002)
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health. We acknowledge the statistical advice of G. Chen and technical assistance from T. Nugent III. The authors thank the children who participated in the study and their families. Author Contributions P.S. designed and wrote the study with J.R. and J.G., and conducted neuroimaging analyses. J.G. and J.R. directed the project. D.G. conducted longitudinal analyses. L.C. was data manager, and R.L. and N.G. advised on interpretation and analysis. J.L. and A.E. developed cortical thickness analytic tools and J.L. developed software for longitudinal neuroimaging analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Notes
This file contains Supplementary Figure 1 (Interaction between age terms and IQ), Supplementary Table 1 (Pearsons’s correlations between IQ and cortical thickness for each cortical region.), Supplementary Table 2 (Demographic and neuroimaging details of subjects), Supplementary Tables 1 and 2 and Supplementary Methods. This file also contains additional references. (PDF 494 kb)
Rights and permissions
About this article
Cite this article
Shaw, P., Greenstein, D., Lerch, J. et al. Intellectual ability and cortical development in children and adolescents. Nature 440, 676–679 (2006). https://doi.org/10.1038/nature04513
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04513
This article is cited by
-
Individual differences in the neural architecture in semantic processing
Scientific Reports (2024)
-
Structural connectome architecture shapes the maturation of cortical morphology from childhood to adolescence
Nature Communications (2024)
-
Parental Deprivation- and Threat-Based Factors Associated with Youth Emotion-Based Neurocircuitry and Externalizing Behavior: A Systematic Review
Research on Child and Adolescent Psychopathology (2024)
-
Individual differences in rhythm perception modulate music-related motor learning: a neurobehavioral training study with children
Scientific Reports (2023)
-
A systematic review and meta-analysis of brain volume abnormalities in disruptive behaviour disorders, antisocial personality disorder and psychopathy
Nature Mental Health (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.