Abstract
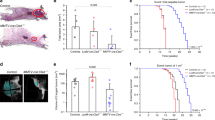
A long-standing hypothesis on tumorigenesis is that cell division failure, generating genetically unstable tetraploid cells, facilitates the development of aneuploid malignancies1,2,3. Here we test this idea by transiently blocking cytokinesis in p53-null (p53-/-) mouse mammary epithelial cells (MMECs), enabling the isolation of diploid and tetraploid cultures. The tetraploid cells had an increase in the frequency of whole-chromosome mis-segregation and chromosomal rearrangements. Only the tetraploid cells were transformed in vitro after exposure to a carcinogen. Furthermore, in the absence of carcinogen, only the tetraploid cells gave rise to malignant mammary epithelial cancers when transplanted subcutaneously into nude mice. These tumours all contained numerous non-reciprocal translocations and an 8–30-fold amplification of a chromosomal region containing a cluster of matrix metalloproteinase (MMP) genes. MMP overexpression is linked to mammary tumours in humans and animal models4. Thus, tetraploidy enhances the frequency of chromosomal alterations and promotes tumour development in p53-/- MMECs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Boveri, T. The Origin of Malignant Tumors (Williams and Wilkins, Baltimore, 1929)
Nigg, E. A. Centrosome aberrations: cause or consequence of cancer progression? Nature Rev. Cancer 2, 815–825 (2002)
Storchova, Z. & Pellman, D. From polyploidy to aneuploidy, genome instability and cancer. Nature Rev. Mol. Cell Biol. 5, 45–54 (2004)
Egeblad, M. & Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nature Rev. Cancer 2, 161–174 (2002)
Lingle, W. L. et al. Centrosome amplification drives chromosomal instability in breast tumour development. Proc. Natl Acad. Sci. USA 99, 1978–1983 (2002)
Pihan, G. A., Wallace, J., Zhou, Y. & Doxsey, S. J. Centrosome abnormalities and chromosome instability occur together in pre-invasive carcinomas. Cancer Res. 63, 1398–1404 (2003)
Jallepalli, P. V. & Lengauer, C. N. Chromosome segregation and cancer: cutting through the mystery. Nature Rev. Cancer 1, 109–117 (2001)
Andreassen, P. R., Lohez, O. D., Lacroix, F. B. & Margolis, R. L. N. Tetraploid state induces p53-dependent arrest of nontransformed mammalian cells in G1. Mol. Biol. Cell 12, 1315–1328 (2001)
Shackney, S. E. et al. Model for the genetic evolution of human solid tumors. Cancer Res. 49, 3344–3354 (1989)
Levine, D. S., Sanchez, C. A., Rabinovitch, P. S. & Reid, B. J. Formation of the tetraploid intermediate is associated with the development of cells with more than four centrioles in the elastase-simian virus 40 tumour antigen transgenic mouse model of pancreatic cancer. Proc. Natl Acad. Sci. USA 88, 6427–6431 (1991)
Galipeau, P. C. et al. 17p (p53) allelic losses, 4N (G2/tetraploid) populations, and progression to aneuploidy in Barrett's esophagus. Proc. Natl Acad. Sci. USA 93, 7081–7084 (1996)
Olaharski, A. J. et al. Tetraploidy and chromosomal instability are early events during cervical carcinogenesis. Carcinogenesis published online, 25 August 2005 (doi:10.1093/carcin/bgi218).
Livingstone, L. R. et al. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell 70, 923–935 (1992)
Artandi, S. E. et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature 406, 641–645 (2000)
Bassing, C. H. et al. Histone H2AX: a dosage-dependent suppressor of oncogenic translocations and tumors. Cell 114, 359–370 (2003)
Carter, S. B. Effects of cytochalasins on mammalian cells. Nature 213, 261–264 (1967)
Uetake, Y. & Sluder, G. Cell cycle progression after cleavage failure: mammalian somatic cells do not possess a “tetraploidy checkpoint”. J. Cell Biol. 165, 609–615 (2004)
Wong, C. & Stearns, T. Mammalian cells lack checkpoints for tetraploidy, aberrant centrosome number, and cytokinesis failure. BMC Cell Biol. 6, 6 (2005)
Boutwell, R. K. The function and mechanism of promoters of carcinogenesis. Crit. Rev. Toxicol. 2, 419–443 (1974)
Shi, Q. & King, R. W. Chromosome nondisjunction yields tetraploid rather than aneuploid cells in human cell lines. Nature doi:10.1038/nature03958 (this issue)
Birchmeier, C., Birchmeier, W., Gherardi, E. & Vande Woude, G. F. MET, metastasis, motility and more. Nature Rev. Mol. Cell Biol. 4, 915–925 (2003)
Daniels, M. J., Wang, Y., Lee, M. & Venkitaraman, A. R. Abnormal cytokinesis in cells deficient in the breast cancer susceptibility protein BRCA2. Science 306, 876–879 (2004)
Yang, X. et al. LATS1 tumour suppressor affects cytokinesis by inhibiting LIMK1. Nature Cell Biol. 6, 609–617 (2004)
Mayer, V. W. & Aguilera, A. High levels of chromosome instability in polyploids of Saccharomyces cerevisiae. Mutat. Res. 231, 177–186 (1990)
Lin, H. et al. Polyploids require Bik1 for kinetochore–microtubule attachment. J. Cell Biol. 155, 1173–1184 (2001)
Feldser, D. M., Hackett, J. A. & Greider, C. W. Telomere dysfunction and the initiation of genome instability. Nature Rev. Cancer 3, 623–627 (2003)
Stukenberg, P. T. Triggering p53 after cytokinesis failure. J. Cell Biol. 165, 607–608 (2004)
Murphy, K. L., Dennis, A. P. & Rosen, J. M. A gain of function p53 mutant promotes both genomic instability and cell survival in a novel p53-null mammary epithelial cell model. FASEB J. 14, 2291–2302 (2000)
Brennan, C. et al. High-resolution global profiling of genomic alterations with long oligonucleotide microarray. Cancer Res. 64, 4744–4748 (2004)
David, G., Turner, G. M., Yao, Y., Protopopov, A. & DePinho, R. A. mSin3-associated protein, mSds3, is essential for pericentric heterochromatin formation and chromosome segregation in mammalian cells. Genes Dev. 17, 2396–2405 (2003)
Acknowledgements
We are grateful to S. Artandi, L. Chin, R. DePinho, C. M. Kuperwasser, M. E. McLaughlin, K. Polyak, A. Protopopov, J. Ruderman, J. Sage, P. Sicinski, T. Stearns and C. Wong for discussions; K. Polyak for showing us procedures with MMECs; A. D'Andrea, R. DePinho, M. Ewen, R. King, M. E. McLaughlin, K. Polyak and Z. Storchova for comments on the manuscript; J. Dunn for assistance with the figures; and S. Doxsey for the anti-pericentrin antibody. T.F. was a Uehara Memorial Foundation research fellow. D.P. was supported by an NIH grant and a scholar award from the Leukemia and Lymphoma Society of America.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Figure S1
Characterization of viable cultures of diploid and tetraploid p53-/- MMECs (PDF 5085 kb)
Supplementary Figure S2
Tetraploid p53+/+ MMECs do not proliferate in vitro. (PDF 3309 kb)
Supplementary Figure S3
Genome-wide array-CGH analysis of tetraploid p53-/- MMECs hybridized to diploid MMECs. (PDF 711 kb)
Supplementary Figure S4
DCB does not induce DNA damage in p53-/- MMECs or wild-type p53+/+ MMECs. (PDF 3282 kb)
Supplementary Figure S5
DNA damage assessed by γ-H2AX labeling in p53-/-MMECs before and after FACS sorting. (PDF 2005 kb)
Supplementary Figure S6
Gross chromosomal rearrangements in tetraploid-derived transformed cells growing in soft agar (Figure 2). (PDF 517 kb)
Supplementary Figure S7
Characterization of the spontaneous tumors derived from tetraploid p53-/- MMECs. (PDF 11442 kb)
Supplementary Figure S8
Gross chromosomal rearrangements in the spontaneous tumors derived from tetraploid p53-/- MMECs. (PDF 301 kb)
Supplementary Figure S9
Genome-wide array-CGH analysis of 8 tumors derived from tetraploid p53-/- MMECs. (PDF 2838 kb)
Supplementary Figure 10
Genes on the amplicons identified from tetraploid-derived tumor. (PDF 356 kb)
Supplementary Figure Legends
This file contains full text descriptions for all Supplementary Figures. (DOC 43 kb)
Rights and permissions
About this article
Cite this article
Fujiwara, T., Bandi, M., Nitta, M. et al. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437, 1043–1047 (2005). https://doi.org/10.1038/nature04217
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature04217
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.